What Is E-Labeling?
E-labeling refers to the electronic distribution of product labeling information. This includes instructions for use (IFU), patient information leaflets (PIL), Summary of Product Characteristics (SmPC), and safety updates via digital channels rather than traditional paper-based inserts.
These can be delivered via QR codes on packaging, dedicated websites, mobile apps, or integration with regulatory systems.
For context, whether it’s a QR code on a box, a tap in a mobile app, or a link from a connected regulatory system, e-labeling transforms labeling from static paper to smart, searchable content.
Why E-Labeling Matters in 2026
Much like most things in modern day life, the future of pharmaceutical labeling is digital.
As the industry faces mounting pressure to cut costs, eliminate waste, and simplify global operations, e-labeling stands out as a smarter, faster, and cleaner solution.
Digital labels make it easier to update content in real-time, ensuring language and region-specific compliance. It also provides end-users with the most accurate and up-to-date information available. Additionally, e-labeling supports environmental sustainability by reducing the need for physical materials, an increasingly important factor in both public and regulatory perception.
Here are some key reasons why you will be hearing more about e-labeling in 2026 and why it’s important:

Key E-Labeling Trends in 2026
E-labeling is leveling up in 2026, and regulators are on board.
E-labeling is becoming more advanced as global regulators are beginning to increasingly support digital labeling solutions. This includes QR codes, Unique Device Identifiers (UDIs), and dynamic multilingual content.
At large, health agencies are releasing new guidance that supports electronic labeling, making it easier to share accurate product information. QR codes and UDIs are now common on packaging, helping connect users to the latest online content.
At the same time, companies are focusing on ways to deliver labels in multiple languages, integrate with regulatory systems, and design content that’s easier for patients to understand.
Below are five key trends shaping the future of e-labeling in 2026.
1. Regulatory Digital Maturity Is Rising
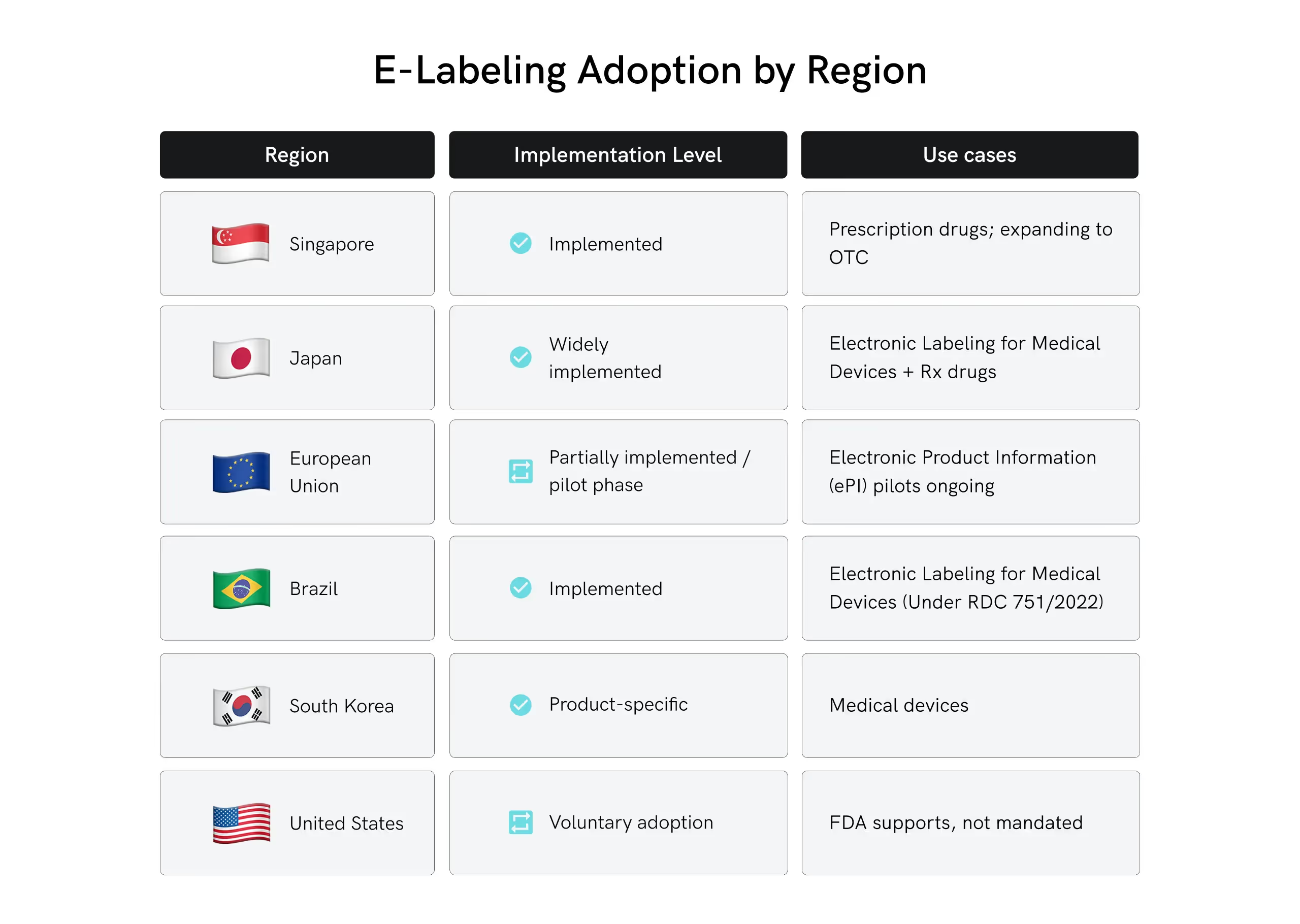
Global regulators are rapidly adapting to digital labeling solution processes. Agencies in the EU, Japan, Singapore, and Brazil have updated guidance supporting electronic labeling for medical devices and medicinal products. In many regions, e-labeling is now recognized as a valid, and sometimes preferred, method of disseminating product information.
2. QR Codes and Unique Device Identifiers (UDI) as the New Normal
QR codes and UDIs are now widely used to link product packaging to up-to-date online information. This trend reflects a broader push toward traceability, supply chain transparency, and improved safety monitoring.
3. Increased Focus on Multilingual and Dynamic Content Delivery
Modern e-labeling systems allow companies to serve dynamic content tailored to the user's language, location, and device. This capability is especially valuable in global markets where paper labels are constrained by space or translation challenges.
4. Integration with Regulatory Information Management (RIM) Systems
E-labeling is becoming tightly integrated with RIM platforms, enabling faster approvals and automatic synchronization between regulatory labeling compliance submissions and public-facing labeling content. This reduces the risk of discrepancies and compliance violations.
5. Patient-Centric Design
The rise of digital health has ushered in a new era of patient empowerment. E-labeling content is increasingly designed for clarity, readability, and accessibility, optimized for smartphones and aligned with health literacy standards.
E-Labeling and Global Regulatory Acceptance in 2026
Regulators around the world are taking steps to define, pilot, and implement e-labeling frameworks.
Some highlights include:
- European Union: The EMA continues to support Electronic Product Information (ePI) initiatives. Several member states have adopted pilot programs, and discussions are ongoing to create a harmonized approach across the EU, especially EU MDR e-labeling requirements.
- United States: FDA e-labeling requirements are becoming more prominent as the FDA has already published guidance on electronic labeling for prescription drugs and devices. While not mandatory, the trend toward voluntary adoption is growing.
- Asia-Pacific: Singapore and Japan are leaders in digital labeling solutions, with national authorities encouraging digital transformation across regulatory systems.
- Latin America: Brazil's ANVISA has made progress in formalizing e-labeling for medical devices, offering clear guidelines on electronic IFUs.
- Middle East & Africa: While still emerging, countries like Saudi Arabia and South Africa are evaluating pilot programs and industry partnerships to bring e-labeling to the region.
Where Has E-Labeling Already Been Implemented?
As of 2026, electronic labeling has been fully or near-fully implemented in a few regions and product categories, though global adoption is still uneven.
Here's a breakdown of where e-labeling has been formally implemented and is actively used in today’s market:
The Future of E-Labeling
E-labeling is no longer a “nice to have”, it’s becoming an essential part of global regulatory labeling compliance and commercial strategy.
In 2026, organizations that embrace e-labeling not only gain operational efficiencies but also position themselves as leaders in patient engagement and regulatory innovation.
As global regulatory acceptance evolves, organizations that invest early in scalable, compliant digital infrastructure, and prioritize the patient experience, will gain a clear competitive advantage and stay ahead of the competition.
FAQs




