What Is Structured Product Labeling (SPL)?
Structured Product Labeling (SPL) is an XML-based document markup standard developed by Health Level Seven (HL7), a non-profit organization that develops standards for the exchange, integration, sharing, and retrieval of electronic health information.
SPLs are required by the FDA for submitting labeling information for human prescription drugs, over-the-counter (OTC) products, biologics, and more.
The SPL XML format structures drug label content in a machine-readable way, ensuring that every section, such as dosage, warnings, and administration instructions, is tagged and organized for both human and electronic use.
The FDA uses SPL to process, validate, and publish labeling information on public databases like DailyMed.
Why SPL Matters: Compliance, Accuracy, and Speed
Every pharmaceutical company marketing products in the U.S. must meet FDA SPL submission requirements.
This is not just a technical format. SPL affects everything from drug label requirements and marketing approvals to digital health compatibility.
Failing to submit accurate, validated SPL can lead to:
- Delays in product approval
- FDA rejection or requests for resubmission
- Fines or warning letters
- Fines
- The FDA has authority under the Federal Food, Drug, and Cosmetic Act (FD&C Act) to impose civil monetary penalties. While fines for SPL-specific violations aren’t always publicized, failure to comply with labeling regulations has led to penalties exceeding $100,000 in some enforcement cases.
- The FDA has authority under the Federal Food, Drug, and Cosmetic Act (FD&C Act) to impose civil monetary penalties. While fines for SPL-specific violations aren’t always publicized, failure to comply with labeling regulations has led to penalties exceeding $100,000 in some enforcement cases.
- Warning letters
- Between 2021 and 2024, multiple companies received FDA warning letters for failing to submit accurate or timely SPL files, especially around misbranded drugs and incomplete labeling. These letters often cite violations of 21 CFR Part 201 and result in public notices that can damage brand trust.
- Risk to patient safety due to inaccurate or outdated information
On the flip side, nailing your SPL submissions ensures:
- Faster approvals
- Fewer manual corrections or validation failures
- Seamless updates to electronic drug labeling systems and platforms
Understanding SPL Data Elements
An SPL file is more than just a digital document. It contains structured metadata and sections that make drug information easy to access and break down.
Key SPL data elements include:
- Product and manufacturer identifiers (e.g., NDC code, company name)
- Labeling version history
- Indications and usage
- Dosage and administration
- Contraindications and warnings
- Drug interactions
- Packaging information
- Marketing status and regulatory info
Each element is tagged in a standardized way, allowing the FDA, and third-party systems, to read and validate the content automatically.
SPL XML File Format
SPLs (Structured Product Labeling) must be in XML format as per strict FDA requirements.
Why XML?
The FDA mandates that SPLs be in HL7-compliant XML format because:
- XML is machine-readable, enabling automated processing and validation.
- It supports semantic tagging of drug information (e.g., dosage, ingredients, warnings).
- It ensures interoperability across different regulatory systems and databases.
It allows for structured and standardized submission, which is critical for labeling, approvals, and updates.
What Must Be in the XML?
The SPL XML must:
- Conform to the HL7 SPL schema.
- Include Logical Observation Identifiers Names and Codes (LOINC) to define label sections.
- Pass validation using FDA’s SPL schema and stylesheet tools.
- Be submitted through the Electronic Submissions Gateway (ESG).
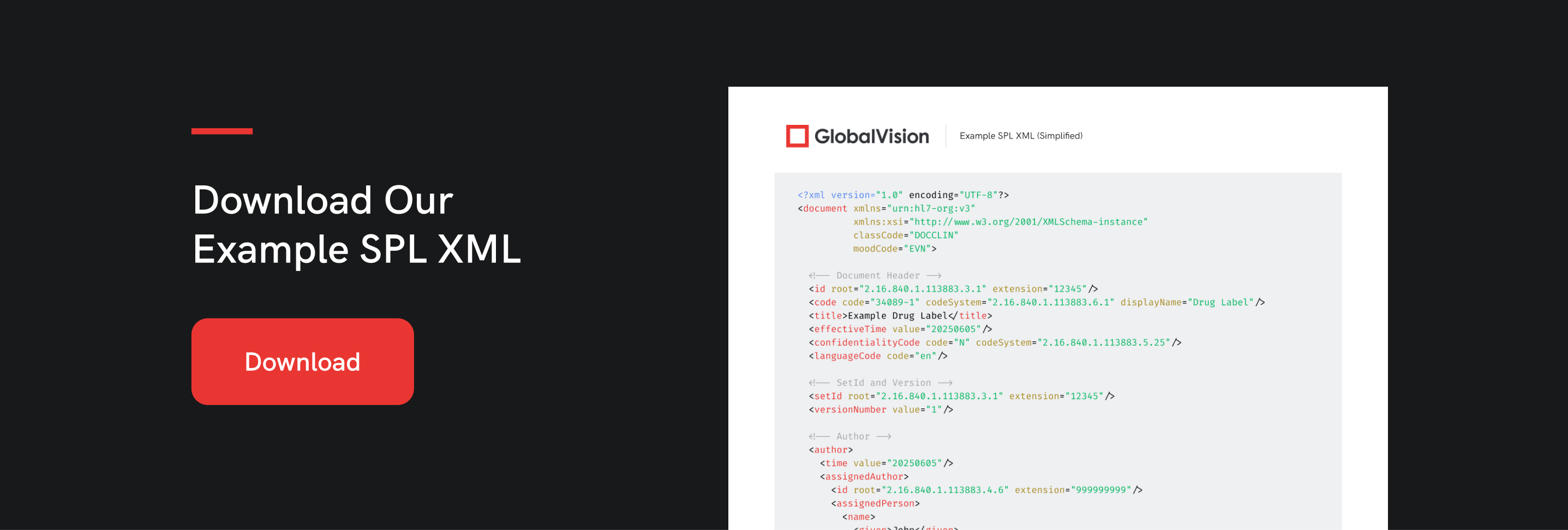
If you are just getting started with SPLs, download our simplified example of an SPL (Structured Product Labeling) XML file format. SPL is based on the HL7 Reference Information Model (RIM) and is used by the FDA to exchange information about drug labels.
Please note, this example is just a basic structure to help you understand the format. Seal SPL submissions to the FDA are significantly more detailed and must comply with strict validation rules.
SPL for Over-the-Counter (OTC) Drugs and Nonprescription Products
Many teams wrongly assume SPL is only for prescription products.
In fact, SPL for over-the-counter drugs is also required if the product is listed with the FDA. This includes vitamins, dietary supplements, and topical agents, among others.
The submission requirements and content structure are similar, although OTC products may have simpler labeling needs. Still, teams must comply with the same standards, which means understanding SPL is essential even outside of Rx products.
FDA SPL Submission Requirements: What You Need to Know
Before submitting your SPL files to the FDA, you need to ensure you are following certain guidelines. Some of them include:
- Proper SPL XML format according to the FDA’s Structured Product Labeling guidance
- A valid FDA ESG (Electronic Submissions Gateway) account for transmission
- Use of the correct validation rulesets provided by the FDA for your product category
- Inclusion of all mandatory data fields and metadata
Additionally, submissions must follow the PLR (Physician Labeling Rule) format for prescription drugs, which changes the sequence and labeling requirements for specific sections.
For a full checklist of submission requirements you need to follow, download our checklist.
Automating SPL Generation: Why Manual Methods No Longer Cut It
Given the complexity of SPL files, and the large risk of human error, many teams are turning to automated SPL generation and SPL compliance software to streamline the process.
Benefits of automating your SPL workflow include:
- Reduced risk of XML formatting or validation errors
- Version control across multiple product labels
- Faster updates when regulations or product details change
- Integration with Regulatory Information Management (RIM) systems
Some tools even provide built-in validation, FDA gateway submission, and change-tracking, making it easier to manage labeling updates across your global portfolio.
The Role of Structured Product Labeling in the Future of Pharma
The push toward electronic drug labeling and data interoperability across healthcare platforms makes SPL more important than ever. As initiatives like IDMP (Identification of Medicinal Products) and global UDI (Unique Device Identification) standards gain traction, structured, digital-first labeling will become the norm.
Pharma teams that invest in SPL knowledge and infrastructure today will be better positioned to:
- Launch products faster in multiple markets
- Avoid compliance pitfalls
- Support digital health integrations
- Enable real-time labeling updates across systems
Your Next Step Toward Smarter Labeling
Structured Product Labeling mastery isn’t just a technical requirement, it’s a strategic capability and competitive advantage.
By understanding the SPL XML format, the FDA SPL submission requirements, and the tools available for automation, your team can stay ahead of compliance risks and regulatory delays.
Whether you're managing updates for a popular drug or submitting a new OTC product, SPL is central to your labeling and go-to-market strategy. And with the rise of automated SPL generation and SPL compliance software, it’s easier than ever to stay compliant, efficient, and competitive.
FAQs