Finding the right pharmaceutical labeling company affects everything from regulatory approval timelines to patient safety outcomes. Unlike standard printing vendors, pharmaceutical labeling partners need to navigate complex regulatory requirements, maintain validated processes, and deliver zero-defect quality under tight deadlines. Choose wrong, and you'll face delayed product launches, compliance risks, or patient safety issues through labeling errors.
Introduction to Pharmaceutical Labeling
Pharmaceutical labels are the critical link between your drug and patient safety. Every dosage instruction, safety warning, and regulatory detail matters because one mistake can trigger recalls, regulatory action, or patient harm.
The pharmaceutical labeling industry includes companies with varying levels of pharmaceutical expertise and regulatory knowledge. The key is finding partners who truly understand drug manufacturing requirements and can integrate smoothly with your existing design workflows.When evaluating partners, choose companies with proven pharmaceutical experience over those treating pharma work as an add-on service. Top pharmaceutical labeling companies operate under cGMP standards, maintain FDA registrations, and employ people trained in pharmaceutical quality systems rather than basic printing operations.
Understanding Pharmaceutical Label Requirements
Your labeling partner needs to understand pharmaceutical regulations inside and out especially when working with FDA-compliant designs generated through systems like Kallik and Veeva.Look for partners who integrate directly with these pharmaceutical design platforms, so your compliant artwork prints exactly as designed. FDA regulations demand specific font sizes for critical information, precise warning placement, and exact reproduction of approved regulatory text. EU guidelines add multi-language requirements and region-specific safety statements.
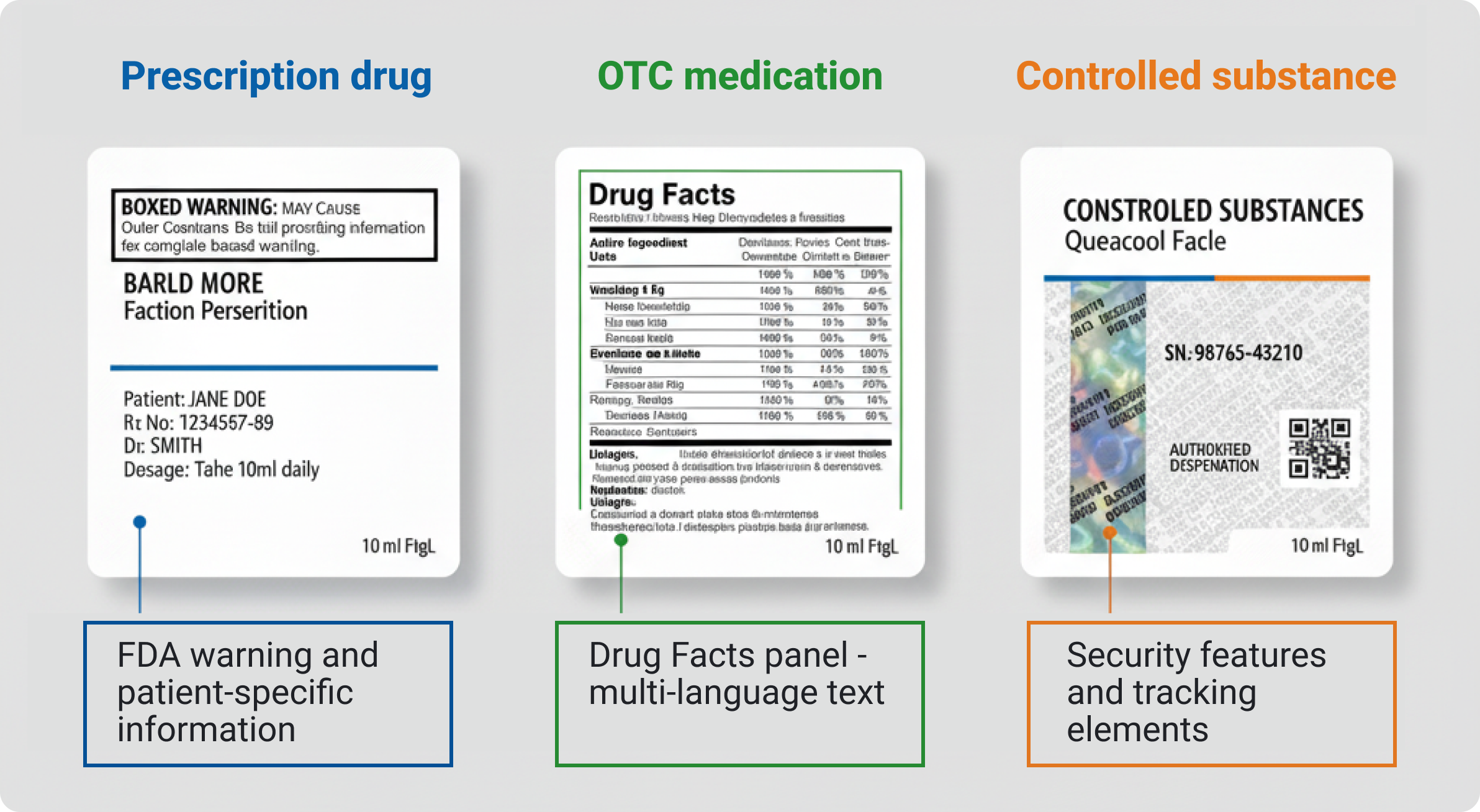
Different products face different rules. Prescription medications operate under stricter standards than over-the-counter products, while controlled substances require security features and tracking capabilities. The best partners walk you through specific examples of how they've managed varying requirements across product categories.
Look for partners who stay current on regulatory changes through dedicated resources, with systems for updating processes when regulations shift and documentation packages that support your regulatory filings. Companies without this regulatory foundation often create risks that can derail product launches through compliance gaps or compromise patient safety through non-compliant labeling.
Choosing the Right Label Materials for Pharmaceutical Products
Material expertise directly impacts product success, making your partner's substrate knowledge critical. Experienced pharmaceutical labeling companies know which materials work under different storage conditions and can recommend the right ones for your products based on the tough environmental challenges pharmaceutical products face throughout their lifecycle.
Refrigerated biologics demand materials that maintain adhesion and readability through cold storage, while ambient temperature tablets need substrates that won't degrade under warehouse conditions. Pharmaceutical applications often require specialized materials beyond basic durability. Tamper-evident substrates show clear signs of interference, protecting patients from compromised products. Temperature-sensitive materials change appearance when exposed to heat, providing visual confirmation that cold chain requirements were maintained.
Companies specializing in pharmaceutical labeling back their material recommendations with actual testing data including accelerated aging studies, adhesion testing, and chemical resistance verification. They should provide this documentation without you having to ask - if they can't show you this comprehensive testing data, keep looking.
Label Safety and Security Features
Anti-counterfeiting capabilities have become increasingly important as pharmaceutical counterfeiting threatens both brand integrity and patient safety worldwide. Your labeling partner needs genuine expertise in security technologies, not surface-level familiarity with basic authentication methods.
Modern security solutions work in multiple layers, with holographic elements and security inks creating visual authentication that patients and healthcare providers can verify, while microprinting and specialized substrates challenge counterfeiters with technical barriers. They should understand how these technologies work together as an integrated security system. RFID and smart label capabilities enable real-time supply chain tracking while supporting serialization requirements, so they need expertise in both physical printing processes and data management systems. Ask potential partners about their experience connecting these technologies with existing tracking systems and request case studies showing actual implementation results. Tamper-evident solutions require careful substrate selection and application techniques that balance security effectiveness with practical usability for legitimate users.
Pharmaceutical Labeling Compliance and Change Control
Change control capabilities separate professional pharmaceutical labeling companies from commercial printers who don't understand regulated environments. Pharmaceutical manufacturing operates under strict change control requirements that your labeling partner needs to understand and support completely. When evaluating potential partners, examine their change management systems to see how they handle documentation requirements while maintaining proper approval workflows. Professional partners provide the validation protocols and impact assessments that keep you compliant while making regulatory submissions easier.
Find companies that actively support regulatory submissions through comprehensive technical documentation and expert testimony when regulatory bodies require additional information. Partners with regulatory affairs expertise help navigate complex approval processes and avoid compliance mistakes that delay your launches. Your partner's change control system should integrate seamlessly with your pharmaceutical quality systems rather than creating additional burden. At the very least, both systems should communicate or offer collaboration capabilities that streamline your workflow. while providing the documentation trail that keeps regulators happy. The right partner makes change control feel natural rather than burdensome.
Specialized Labeling Solutions for Clinical Trials
Clinical trial labeling requires specialized capabilities that standard pharmaceutical labeling can't address, so if your pipeline includes clinical products, verify that potential partners have validated processes for these complex requirements. Blinding techniques ensure study integrity by concealing treatment assignments from participants and investigators while maintaining randomization protocols through specialized printing processes and quality controls. Ask potential partners for specific examples of blinding solutions they've implemented along with the validation documentation that supports these processes.
Randomization support and multi-site trial coordination demand variable data printing capabilities that assign unique identifiers while maintaining study protocols across global locations. This requires solid data management systems and quality controls that protect patient confidentiality while maintaining the audit trails regulators expect. Make sure your partner can manage sensitive clinical data without risk - confidentiality should be built into both their systems and workflows.
Advanced Functionality in Pharmaceutical Labels
Extended content labels and peel-and-reseal technologies solve space constraints while providing comprehensive patient information, making them particularly valuable if your products require extensive safety information or patient education materials. Smart label features like QR codes are increasingly important as e-labeling gains regulatory acceptance, particularly in the EU with growing adoption in the US. These QR codes typically link to electronic versions of labels, allowing manufacturers to provide comprehensive product information while meeting space constraints on physical packaging. Near field communication (NFC) and augmented reality can improve patient education and still meet regulatory compliance.
The key is ensuring they connect with existing patient support programs instead of adding new layers or creating disconnected workflows. Ask potential partners about their experience implementing advanced functionality and request performance data showing they work reliably throughout your product's life. Advanced features require careful validation to ensure consistent performance under real-world conditions, which makes innovation capabilities important when evaluating long-term partnerships. Look for companies investing in new technologies and demonstrating thought leadership in pharmaceutical labeling advancement rather than those simply following industry trends.
Pharmaceutical Packaging Types and Label Applications
Your partner should demonstrate expertise across the packaging formats relevant to your product portfolio because each packaging type presents unique labeling challenges.
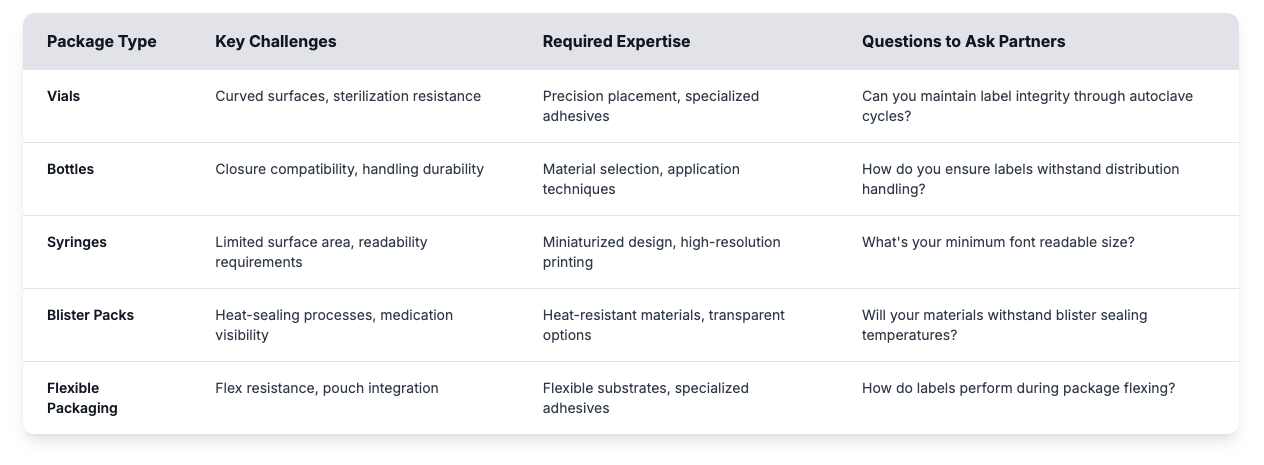
Evaluate potential partners' experience with your specific packaging types by requesting examples of similar applications and performance data demonstrating successful implementations under real-world conditions. Specialized pharmaceutical partners understand how to match substrates, adhesives, and durability needs to your product.
Environmental Sustainability in Pharmaceutical Labeling
Sustainability capabilities are shifting from corporate nice-to-have features toward regulatory expectations that can impact product approval and market access in many markets. Pharmaceutical labeling specialists invest in eco-friendly solutions that maintain performance standards while supporting your environmental commitments. Recyclable substrates and biodegradable adhesives can now match the performance of traditional materials, but choosing the right ones takes a clear understanding of both environmental impact and pharmaceutical requirements. Your labeling partner should understand how these materials perform across your storage conditions and provide lifecycle data that supports both sustainability reporting and regulatory compliance.
Label optimization reduces environmental impact through efficient layouts that maximize substrate utilization and multi-functional designs that eliminate secondary packaging components while maintaining the information density regulatory bodies require. The best partners don't just talk sustainability - they prove it with real data and actions. They know where their materials come from and track the energy their production uses. When products reach end-of-life, they have disposal plans that actually work. Skip the companies making broad environmental claims without the data to back them up. Look for partners who can show their carbon footprint numbers, prove they've cut waste, and explain how they plan to keep improving.
Companies specializing in pharmaceutical labeling provide carbon footprint data, sustainability certifications, waste reduction achievements, and continuous improvement plans that support your corporate sustainability goals while maintaining the product safety and regulatory compliance that patient care demands.

Quality Assurance in Pharmaceutical Labeling
Quality assurance capabilities separate companies that print pharmaceutical labels from those that truly understand pharmaceutical manufacturing. The difference shows up in their approach to error prevention, their inspection system sophistication, and whether their quality documentation actually supports your regulatory requirements instead of creating more work. Automated verification technologies have become the preferred approach for pharmaceutical labeling quality control, comparing text, graphics, barcodes, and spelling against approved content with precision that manual review can't match.
cGMP compliance in label manufacturing means maintaining environmental controls for consistent conditions, qualifying equipment for reproducible results, and training personnel in pharmaceutical manufacturing principles beyond basic printing knowledge. When evaluating potential partners, verify they maintain proper facility registrations and quality certifications, and observe how these requirements translate into daily operations through their quality control processes - incoming material inspection, in-process monitoring, and finished product verification. The documentation from these processes should integrate smoothly with your pharmaceutical quality systems.
Partnering With Pharmaceutical Labeling Companies
Financial stability and business continuity matter because labeling delays can shut down your entire manufacturing operation. Check potential partners' financial health, facility backup plans, and how they handle emergencies that could disrupt your supply chain.
Key questions for potential suppliers include:
- Do you maintain FDA registration and current cGMP compliance certification?
- Which quality certifications do you hold (ISO 9001, pharmaceutical-specific certifications, etc.)?
- Are customer references available from similar pharmaceutical applications?
- Tell us about your emergency production capabilities and response times.
- How does your change control system integrate with pharmaceutical quality systems?
- How do you stay current with regulatory changes and what validation documentation do you provide for submissions?
- Can you scale production to meet our volume requirements?
- Do you support labeling for our target markets globally?
Discuss their material capabilities, printing technologies, and how their quality systems work with your existing processes instead of against them.
Strong partnerships don't happen by accident - they take real work from both sides. You need regular check-ins, performance tracking, and honest communication to make things work. The best partners actually care about your success, not just cashing your checks. When you're working out contract details, get clear on what quality looks like, who handles what regulatory responsibilities, and how you'll measure performance. Build in room for change - whether that means adapting to new regulations, responding to emergencies, or scaling as your business grows.
The Future of Pharmaceutical Labeling
Serialization requirements continue expanding globally as more countries implement track-and-trace mandates, making these capabilities essential for pharmaceutical labeling partners today. AI-driven inspection tools are already reshaping labeling workflows with automated quality checks and real-time error detection. Major pharmaceutical companies are developing content and labeling automation roadmaps that use AI agents to handle key tasks, moving from manual processes to intelligent automation. and built-in quality checks. E-labeling adoption will speed up as regulators become more comfortable with digital labels. The EU is leading the charge while the US gradually follows with its own digital requirements. AI will move beyond just catching errors to actually creating content, checking regulatory compliance automatically, and managing workflows without human input.Partners should show they understand these industry shifts and have concrete plans for adapting to AI-powered requirements rather than vague promises about staying current with technology.
Case Studies: Successful Pharmaceutical Labeling Solutions
Two pharmaceutical companies faced different labeling quality challenges but found similar solutions through automated inspection technology.
Case Study 1: iNova Pharmaceuticals - Eliminating errors and accelerating launches
Operating across 20+ countries, iNova Pharmaceuticals has products ranging from weight management to dermatology. They needed to create an artwork process that worked consistently across all three regions without relying on external studios where they had little control. Bringing GlobalVision on board let them take back ownership of their artwork management workflows and get first-hand accuracy of their files. The result? Faster, higher-quality revisions and better speed-to-market for new product launches. See how iNova transformed their quality process
Case Study 2: Johnson & Johnson’s Ethicon - Scaling quality reviews globally
Johnson & Johnson’s worldwide labeling department was stuck doing tedious manual comparisons between all file revisions. The GlobalVision software brought simplicity to their proofreading by discarding intended changes and flagging critical or unintended changes for investigation. Since implementation, they’ve saved considerable time running quick file inspections while gaining peace of mind that their latest working version is error-free. The outcome: consistently accurate packaging and labeling that eliminates the risk of content and artwork errors. Read the full Johnson & Johnson case study.
These examples show that whether you’re bringing processes in-house or scaling existing operations globally, selecting partners with advanced inspection capabilities delivers measurable improvements in both speed and accuracy.
Technical Support and Customer Service Considerations
Technical support matters because labeling problems can shut down your entire manufacturing operation. You need to know your partner can actually help when things go wrong, not just promise good service in their marketing materials. When you're looking at the support capabilities of pharmaceutical partners’ keep these essential factors in mind:
Technical Support Structure:
- Account managers who actually understand pharmaceutical work
- Technical experts you can reach when problems come up
- Response times that work for your operations
- Clear ways to escalate urgent problems
Emergency Production Capabilities:
- Backup plans when supply chains break down
- Extra production capacity for when things get critical
- Access to backup facilities so you don’t get shut down
- Rush order handling and tight deadline management
Training and Education Services:
- Getting your team trained and comfortable with new systems
- Keeping everyone updated when regulations shift
- Showing your team new tools that might make their jobs easier
- Giving your team resources they can look back at when they need help
The right pharmaceutical labeling company becomes a strategic partner in your quality assurance program, understanding your regulatory environment while providing technical expertise needed to maintain compliance as requirements evolve.
Ready to see AI-powered inspection technology in action? GlobalVision's Verify catches labeling errors before they become costly recalls.



