When a packaging company ships labels with incorrect barcodes or a printer delivers cartons with color variations that don’t match brand standards, the fallout goes far beyond immediate reprints. Brand owners lose trust. Retailers start rejecting shipments. Meanwhile, competitors gain shelf space while your reputation tries to recover.
Production quality control stops these disasters by catching problems before they reach customers. The most successful print and packaging operations don't treat quality as an afterthought or rely on final inspection to catch defects. They build quality control into every stage of production, creating systems that identify issues early and prevent them from recurring.
Understanding Production Quality Control in Print & Packaging
Production quality control catches defects before they become customer problems. In print and packaging operations, this means ensuring that what comes off the press matches approved digital files and brand specifications. While quality assurance focuses on preventing defects through process design and prepress workflows, quality control detects and corrects problems during the printing process and post-press finishing. Every print production process introduces variation from substrates and inks, press performance changes, environmental conditions like humidity and temperature, and press operators. Quality control identifies when these variations threaten product quality and provides the data needed to fix problems.
Modern print and packaging operations have evolved beyond relying solely on final inspection to catch quality issues. Traditional quality control focused heavily on end-of-run detection, which was both expensive and ineffective at preventing customer problems. Today's systems integrate quality checks throughout the entire production workflow - from pre-production proof verification through in-process monitoring during press runs to final validation. This evolution happened because print and packaging operations learned a fundamental truth: preventing defects through early detection and process control costs far less than fixing problems after they reach customers. The best quality control systems now work alongside production at every stage rather than waiting until the end.
Quality Control vs. Quality Assurance: Key Differences
Quality assurance (QA) and quality control (QC) do different things in print and packaging operations, but they need to work together for your quality management system to actually function. QA and QC require different skills and tools, yet they have to coordinate closely if you want consistent product quality. Quality assurance provides the foundation by creating print production processes designed to prevent problems, while quality control provides the safety net by catching problems that occur despite preventive measures.
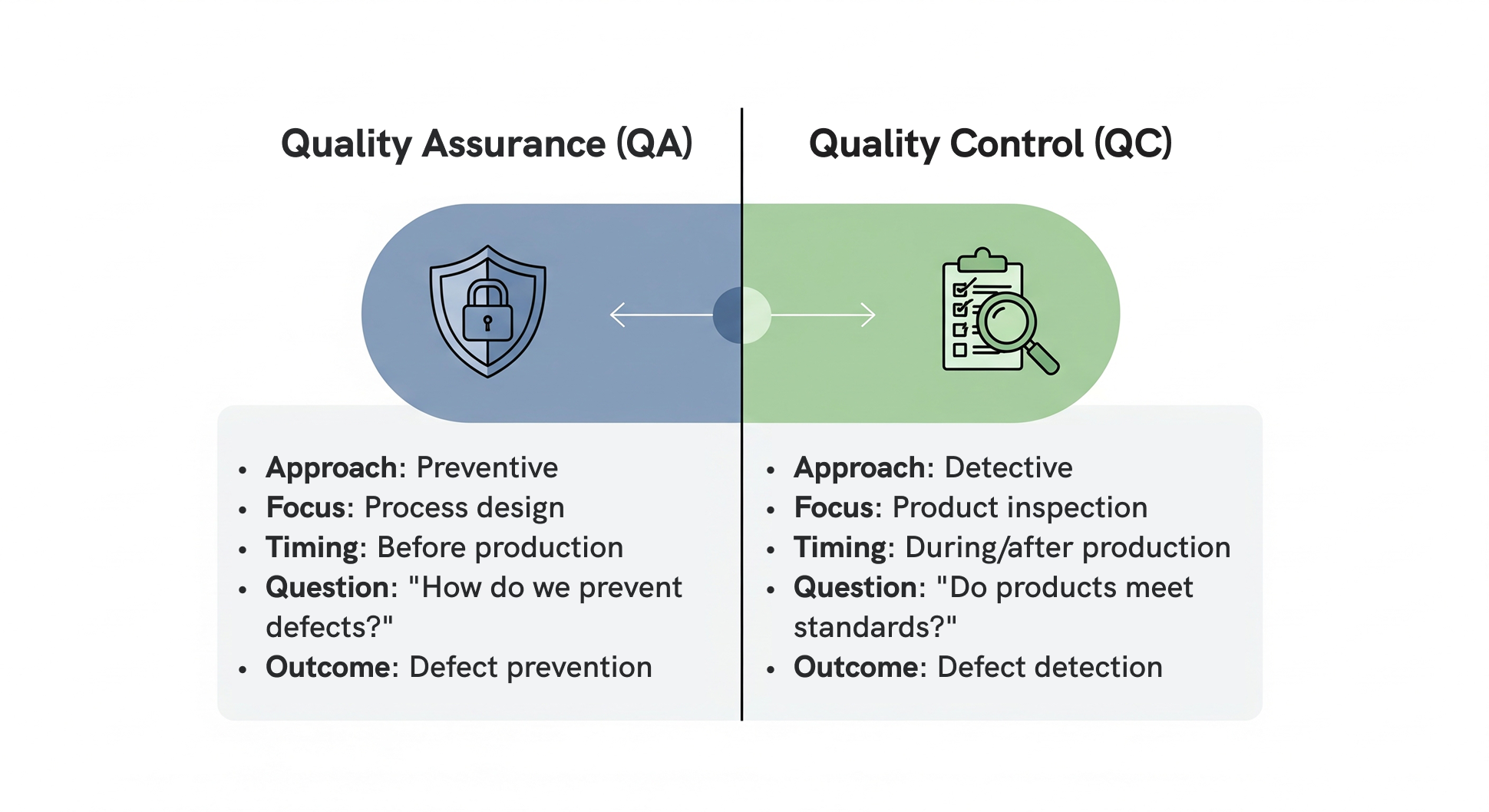
Quality assurance focuses on prevention. It’s designing prepress workflows that work, qualifying substrate and ink suppliers you can trust, and keeping press equipment running smoothly. Quality control catches problems after they happen t
hrough inspection, testing, and measurement to make sure your printed materials meet brand standards.
While these functions use different tools and skills, the best quality management systems make them work together instead of keeping them separate. Quality assurance relies on process audits and training programs, while quality control depends on inspection equipment and sampling techniques.
The Critical Importance of Quality Control in Print Production
Quality control protects your most valuable business assets. Brand reputation depends heavily on product quality - a single quality incident can damage a reputation that took years to build. Print production operations that maintain consistent quality command premium pricing and enjoy higher customer loyalty.
The financial impact extends throughout your operation. Reduced defect rates decrease waste, rework costs, and warranty claims. Print production operations that maintain consistent quality spend less time fixing problems and more time producing quality printed material, translating directly into lower unit costs and higher profitability.

Regulatory compliance adds another critical layer. Pharmaceutical packaging faces strict quality requirements backed by significant penalties for non-compliance. Consumer goods - including food, beverages, cosmetics, and cleaning products - deal with similar pressures. Quality control ensures you meet regulatory standards while avoiding costly recalls along with expensive fines and legal liability. Quality problems create risks that go beyond regulatory compliance. When things go wrong, production lines shut down. Emergency customer audits get triggered. Your most supplier relationships take a hit. That’s why effective quality control systems matter - they catch potential risks before you’re dealing with expensive operational disasters.
Essential Quality Control Methodologies
Three proven methodologies form the backbone of effective quality control programs. Each serves a different purpose, but the most successful print and packaging operations combine all three to create quality systems that actually work.
Total Quality Management (TQM) makes quality everyone's responsibility rather than limiting it to a single department. This approach emphasizes customer focus, continuous improvement, and employee involvement throughout the organization. Making TQM work requires real cultural change that goes beyond technical procedures. Management must show commitment through resource allocation and decision-making. Employees need training and authority to identify and fix quality issues. Suppliers must become partners in quality improvement rather than vendors competing solely on price.
Statistical Quality Control (SQC) gives you the analytical tools for data-driven quality decisions. It uses statistical methods to monitor process performance, identify trends, and predict when processes may go out of control. Control charts are the most common SQC tool, showing process data over time to reveal patterns and variations. Statistical process control builds on SQC by using statistical methods to control processes in real-time. Instead of monitoring quality after production, statistical process control helps operators keep processes within acceptable limits during production. This approach prevents defects rather than just catching them.
Six Sigma methodology focuses on defect reduction through structured problem-solving. These projects follow the DMAIC (Define, Measure, Analyze, Improve, Control) process to systematically find and eliminate sources of variation. The goal is reducing defects to fewer than 3.4 per million opportunities. The methodology's strength comes from its data-driven approach. Projects use statistical analysis to find the real causes of problems and prove that fixes actually work. This focus on data helps make sure improvements last and deliver results you can measure.
These methodologies give you the strategic framework, but making them work requires smart decisions about inspection methods.
Quality Control Inspection Methods
Choosing the right inspection approach is one of the most critical decisions in print production quality control system design. You need to balance thoroughness with cost, while considering speed based on your press requirements and brand risk tolerance.
When using sampling techniques, acceptance sampling plans define how many units to inspect and the criteria for accepting or rejecting entire production lots. These plans provide statistical confidence in quality decisions while reducing inspection costs and time. Sample sizes must be large enough to detect defects at specified confidence levels, and proper sample selection ensures samples represent the full production lot.
100% inspection makes sense for critical packaging components, high-value print runs, and anything with strict regulatory requirements like pharmaceutical labeling or food packaging. You get complete confidence that no defects reach brand owners or consumers. Pharmaceutical packaging, luxury brand materials, and safety critical labeling operations use this approach.but expect higher costs, slower production, and potential damage from extra handling.
Automated inspection works great for high-volume print production where you're looking for the same types of defects over and over - color variations, registration issues, text clarity, and barcode readability. These systems give you fast, consistent results that keep up with production speed. High-volume package operations, label production and commercial printing facilities love them but setup costs are high and they miss complex problems that need human eyes.
Manual inspection might come up for prototypes or one-off specialized products, but automated systems deliver superior consistency and speed in production environments. They’re also far more accurate.
Hybrid approaches mix different methods based on what matters most for each print component.You'll see this in packaging operations, commercial printing, and label production where some parts are critical (like barcodes) and others aren't (decorative graphics). Just expect more coordination between different inspection teams.
The most effective print production quality control systems combine multiple inspection approaches strategically. Critical characteristics like barcodes might receive 100% automated inspection, while less critical features like decorative graphics could be monitored through sampling.
These inspection activities generate the data that powers modern quality control programs, giving you the foundation for making smart decisions about your operation.
Data-Driven Quality Control Programs
Print production data contains the insights you need to understand quality performance and identify improvement opportunities. To make this data useful, quality data collection must be structured and consistent. This means capturing color measurements, barcode verification results, text inspection findings, and press parameters ways that quality control specialists and press operators can actually access and use.
Raw data doesn't tell you much on its own. Key performance indicators (KPIs) turn print quality data into useful metrics that show how you're doing against quality goals. The most useful print quality KPIs include color deviation rates, along with first-pass print yield, barcode compliance rates. Cost of poor quality rounds out the essential metrics. All of these directly connect to business results and customer satisfaction.
The real power comes from acting on data quickly. Real-time monitoring lets press operators fix quality issues immediately instead of discovering problems after thousands of bad prints come off the press. Trend analysis over time also helps - you may notice gradual color drift, humidity causing problems during certain seasons, or equipment wear affecting print quality.
Making data actionable requires the right presentation. Data visualization tools make complex print quality information accessible through control charts and trend graphs, plus dashboards that help press operators and managers quickly understand quality performance. Important data points to track periodically include:
- Most common barcode and QR code defects (such as quiet zone violations and bar width reduction)
- How often text and graphics issues show up on different substrates
- How many inspections you’re running per production batch
- Whether braille dot height meets industry standards
- How much color varies from what your brand requires (delta E measurements)
-
Using these specific data points helps print operations adjust processes and reduce errors systematically over time. When you integrate quality data with production schedules, substrate lot numbers, and press maintenance records, you start seeing hidden relationships that affect print output in ways you never expected. The best analytics platforms also work with your existing workflow management systems, giving you one complete picture of print quality across your entire operation.
Implementing an Effective Quality Control Plan
Building a quality control strategy that actually works takes planning that connects your quality goals with what your business needs. To build a quality control plan that works, focus on these four areas:
- Set realistic quality standards: You need to know what brand owners and customers actually want, then turn those expectations into specific print specifications you can measure. Make sure these specifications are clear, doable, and everyone in your organization understands them. Your quality requirements should cover print characteristics like color accuracy, registration tolerances, and substrate handling that actually matter to your business. Find the right balance between what customers want and what you can actually produce - overly tight color tolerances drive up production costs without improving customer satisfaction, while loose tolerances create quality issues that damage brand relationships
- Document procedures that people actually follow: Documentation forms the backbone of effective quality control systems, but only if press operators can find and use it. Quality procedures must be clearly written, regularly updated, and easily accessible to all personnel. Work instructions should provide step-by-step guidance for quality control activities, including inspection methods, acceptance criteria, and non-conformance procedures. Your quality control procedures need to cover everything from checking incoming substrate and inks to verifying finished printed materials.
- Train your team effectively: People need to understand both what to do and why it matters. Training should cover technical skills and quality awareness, helping employees see how their actions affect print quality. Regular refresher training keeps skills current and reinforces quality expectations throughout the print operation.
- Coordinate across departments: Quality control only works when everyone's on the same page. Press teams need to understand quality requirements, maintenance needs to keep inspection equipment calibrated, and purchasing has to ensure substrate suppliers meet quality standards. Without this coordination, even the best quality plan falls apart.
Effective implementation also depends on having the right tools and techniques to execute your quality control plan successfully.
Essential Quality Control Tools and Techniques
Process capability analysis shows you whether your print production processes can actually meet quality requirements consistently. This technique compares how much your press varies against your specification limits to see if you can produce consistent packaging and labels reliably. Capability studies help print operations identify processes needing improvement and establish realistic quality targets for color accuracy, registration tolerance, and print consistency.
Capability indices like Cp and Cpk quantify print performance in easy-to-understand metrics that help press operators communicate results to brand owners, packaging suppliers, and internal stakeholders. Regular studies track improvement over time and identify when processes begin to deteriorate.
Two proven methods dominate root cause analysis in print product:

When print quality problems do occur, root cause analysis methods help you identify and eliminate the underlying causes rather than just treating symptoms. The 5 Whys technique and Fishbone diagrams provide structured approaches to problem-solving that prevent issues from recurring.
The 5 Whys technique works well for straightforward problems with clear cause-and-effect relationships. For example, when color consistency issues appear on-press, you might keep asking ‘why’ and discover the real issue isn’t the ink temperature but a missing preventative maintenance program for color management systems that was never prioritized by management.
Fishbone diagrams help with complex problems where multiple factors contribute to quality issues. A print defect investigation often uncovers problems across multiple areas. You might find issues with your materials - maybe ink quality is inconsistent or substrate prep was rushed. Sometimes it’s your methods, like wrong press settings or color profiles that don’t match. Machine problems show up too: print heads wearing out or registration systems drifting. Don’t forget the human factor - operators might need better color matching training. Even environmental stuff matters, like humidity messing with ink flow. And sometimes your measurement tools just need calibration. The visual layout helps press teams think through all possible causes instead of jumping straight to obvious answers.
Most print operations do better when they use both methods together - use Fishbone diagrams to map out all potential causes, then hit the most likely ones with 5 Whys to find the real problem. Behind all these tools, measurement system analysis ensures your inspection and testing equipment provides accurate and reliable results. Even the best quality control procedures fail if measurement systems are unreliable through poor calibration or gauge repeatability issues.
Technology Integration in Modern Quality Control
Computer vision systems have transformed visual inspection in print production environments. These AI-powered systems detect print defects, measure color accuracy, and verify barcode quality faster than press operators can manage. This is especially true for high-speed, repetitive inspections that would challenge human operators. In print production specifically, file-to-sample comparison technology automatically compares digital files against what actually comes off the press. High resolution scanning with built-in sensors catches differences with pixel-level accuracy that traditional inspection would miss entirely.
Making computer vision work in print environments requires careful consideration of lighting conditions and sensor positioning, plus image processing algorithms optimized for print substrates. Different print defects need different inspection approaches, and systems must be configured properly to catch real quality issues like color variations and registration problems while avoiding false alarms from acceptable substrate variations. Getting these systems right for print operations often means working with integrators who understand both the technology and your specific press configurations.
IoT sensors take print quality monitoring to the next level by tracking press parameters that affect print quality in real-time. Temperature and humidity get monitored continuously, along with ink viscosity, and press speed. When conditions drift outside acceptable ranges, you get immediate alerts. This catches quality issues before they happen rather than after printed materials are already produced. The real advantage comes when you connect IoT data with your quality control systems. Analyzing how press parameters affect quality outcomes lets you predict when defects are likely to occur. Instead of reacting to problems, you can prevent them from happening in the first place.
Artificial intelligence and machine learning are reshaping print quality control by uncovering patterns in quality data that traditional methods can’t detect. These systems get better over time by learning from inspection results and press data, then factoring in maintenance records to spot print quality problems early. AI can process all this data at once and identify patterns that show how different variables affect print output quality.
For Continuous Improvement Managers overseeing these implementations, effective technology adoption requires investment in training and infrastructure, backed by proper support systems. Smart print facilities plan technology integration carefully and implement gradually to avoid disrupting production workflows. The payoff comes when these tools start preventing print quality problems instead of just catching them after production. The key is starting small with one technology that solves your biggest print quality challenge, then building from there as you see results.
Continuous Quality Improvement Strategies
Kaizen philosophy focuses on continuous, incremental improvements that add up to significant quality gains over time. Instead of waiting for major problems to trigger improvement projects, Kaizen makes quality improvement part of daily print operations. This approach empowers employees to identify and implement improvements because workers closest to press processes often have the best insights into what needs fixing.
The PDCA (Plan-Do-Check-Act) cycle gives you a structured framework for quality improvement projects. This approach includes root cause analysis during planning, small-scale testing during implementation, thorough evaluation of results, and standardization that makes successful improvements permanent parts of the quality control system. The cycle repeats continuously, driving ongoing quality enhancement.
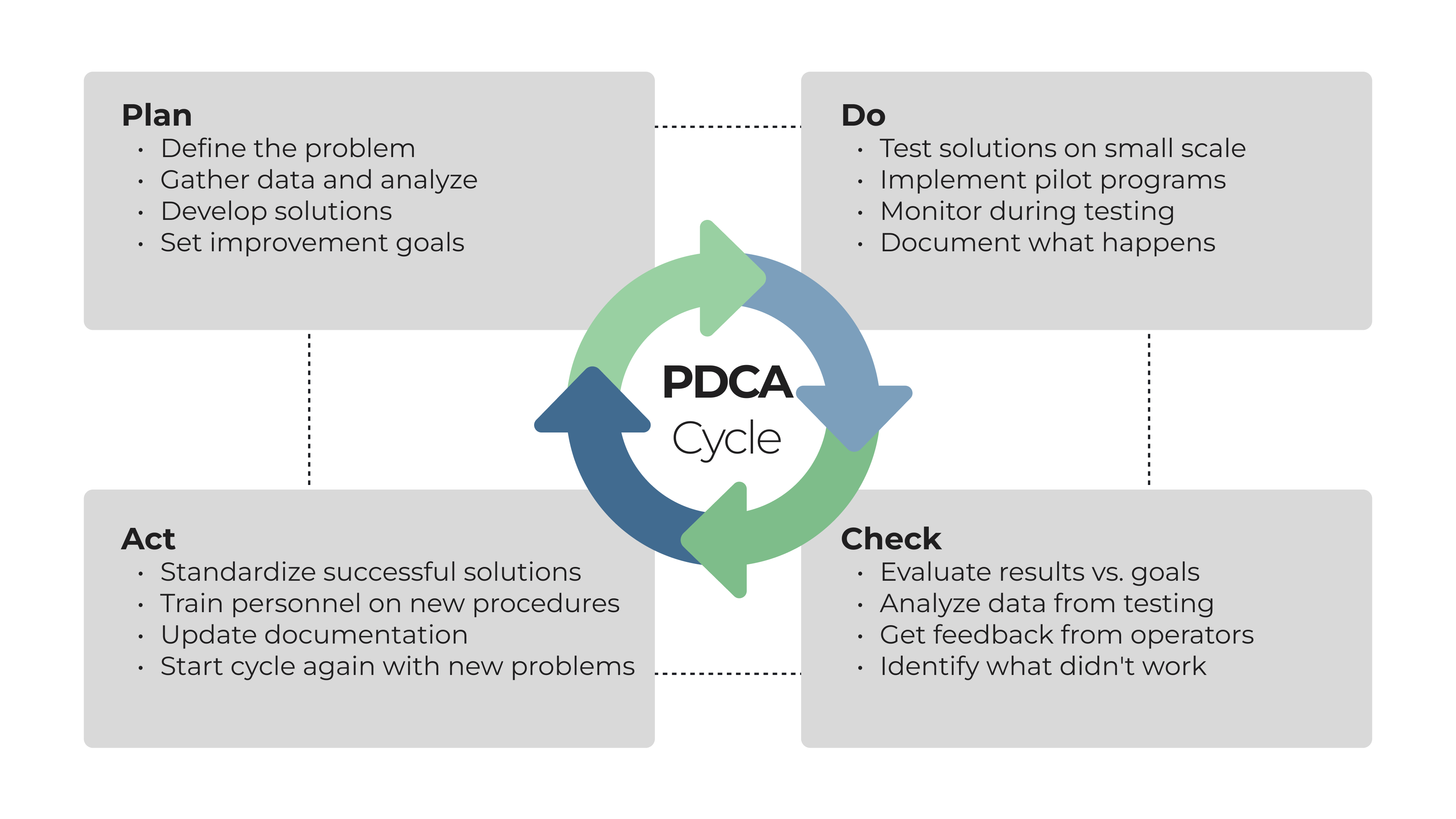
Creating a culture of continuous improvement needs leaders who are committed and employees who are engaged. Management must show that quality is a genuine priority through resource allocation and decision-making. Employees need training, tools, and authority to address quality issues effectively. Without this cultural foundation, even the best improvement methodologies fail to deliver lasting results. Keep everyone engaged by sharing quality wins and talking openly about challenges that still need solving.
Measuring ROI and Cost Considerations in Quality Control
Calculating the true cost of poor quality shows how much quality problems actually cost and explains why investing in quality control systems makes sense. Most print and packaging operations underestimate these costs because they only track obvious expenses like reprints and substrate waste while missing hidden costs that are often much larger.
Quality problems cost you money in two ways:
- Direct costs (the obvious ones):
- Reprints and substrate waste
- Customer rejections and chargebacks
- Press downtime
- Indirect costs (harder to track, but usually bigger):
- Lost contracts from quality issues
- Customer acquisition cost (CAC)
- Brand reputation damage
- Emergency shipping and expediting
Smart quality control investments balance costs against business outcomes. Quality control systems cost money upfront for equipment and people. Add in procedures, and the initial investment adds up. But they pay you back through fewer defects, better efficiency, and happier customers. When you calculate ROI, look at both immediate benefits like reduced reprint costs and long-term benefits like improved brand reputation and customer loyalty.
Key performance indicators prove whether your quality control programs actually work. Financial metrics like cost of poor quality and quality-related savings give you hard numbers to show management the program's value. When the cost of poor quality decreases over time, it demonstrates your program is working. Operational metrics like defect rates and first-pass yield show process improvements in real-time - declining defect rates and increasing first-pass yield prove your quality control systems are effective.
Making metrics work starts with clear definitions, and consistent measurement, then regular reporting to keep everyone informed. Dashboard reporting systems make quality performance visible throughout the organization and enable rapid response to problems. Without regular review of these metrics, you miss trends and improvement opportunities that could save significant money.
Overcoming Common Quality Control Challenges
Complex supply chains make quality control challenging because you're dealing with multiple stages from prepress through finishing across different departments and locations. Every step can introduce quality problems, and trying to catch everything through inspection alone becomes overwhelming and ineffective. The answer is building real partnerships with suppliers who share your quality standards instead of treating them like adversaries you need to police.
Supplier qualification programs establish minimum quality requirements and verify suppliers can meet them consistently through facility audits, process evaluations, and ongoing performance monitoring. Strong supplier relationships make quality improvement easier and help everyone win big.
Human factors significantly influence quality control effectiveness. Even well-designed procedures fail if operators lack proper training, motivation, or tools. Smart quality systems account for human limitations and support good performance rather than fighting against it. Training programs need to address both technical skills and quality awareness. Operators should understand how to perform quality control activities and why these activities matter to the business. Regular refresher training keeps skills current and reinforces quality expectations throughout the print operation.
When selecting quality control software, user-friendly programs keep production running smoothly and get teams up to speed fast. Complex interfaces slow down implementation and frustrate operators who are trying to learn unfamiliar systems.
Short-run printing and custom packaging environments create unique quality control challenges. Traditional statistical techniques don’t work when production lots are small and jobs change frequently. These environments need flexible quality control approaches that adapt fast to different products and volumes. Modular quality control systems that reconfigure easily for new jobs provide one solution. Standardized inspection procedures that work across multiple product lines reduce setup time and training requirements. Investment in flexible automation delivers consistent quality control across diverse print operations.
Fast-paced production environments need quality control that can match the speed without cutting corners. Automated inspection and real-time monitoring give you quick feedback so operators can fix problems immediately. Statistical sampling cuts inspection time while keeping you confident in quality.
These challenges aren't going away, but new technologies are creating better ways to handle quality control.
Future Trends in Print Production Quality Control
Industry 4.0 - the Fourth Industrial Revolution integrating digital technologies like IoT, cloud computing, AI, and robotics - is changing how quality control works. Instead of isolated quality systems, Industry 4.0 connects quality control with production planning, maintenance management, and supply chains. This integration lets print and packaging operations optimize their entire operation instead of managing each piece separately.
Predictive quality analytics represents a major change in approach. These AI-powered tools analyze historical data and use machine learning algorithms to spot quality problems before they develop. You can prevent issues instead of scrambling to fix them afterward. Building these capabilities requires investment in data collection systems, and storage infrastructure. You’ll also need analytical tools. But the payoff comes through fewer quality headaches and better products.
Cloud computing enables real-time quality data sharing across multiple facilities and departments. IoT sensors throughout production lines feed continuous quality data to centralized systems. This connectivity means quality managers can monitor operations remotely and respond to issues immediately, regardless of location.
Augmented reality (AR) is creating new opportunities for quality control training and execution. AR systems walk operators through complex inspection procedures step-by-step, reducing errors and speeding up training.
Blockchain technology provides secure, unalterable quality records that improve traceability and compliance. Advanced sensors can monitor quality parameters that were impossible to measure before.
The most successful print and packaging operations will build flexible, data-driven quality systems that combine human expertise with these new technological capabilities. Technology won't replace experienced quality professionals - it makes them more effective. Success comes from integrating new tools with human judgment to deliver consistently high-quality products that meet customer expectations.
Want to catch defects before they reach your customers? Check out GlobalVision's GVD platform for reliable automated inspection.