Introduction to Pharmaceutical Artwork Management
In pharma, artwork isn’t just cosmetic. It’s how patients get the right dose, and how companies show regulators they’re following the rules. If labels are wrong, or out of date, the risks aren’t small. Recalls, penalties, and patient harm can all follow.
Modern artwork management helps teams handle this risk-heavy process with more control. A solid process keeps teams aligned and errors out of the system. It connects regulatory, marketing, supply chain, and quality teams around a clear, controlled artwork development process. When done right, it reduces human errors, prevents the delays caused by excessive revision cycles, and accelerates time-to-market through parallel approvals, automated workflows, and fewer revision cycles that eliminate launch delays.
From Paper to AI: Pharma Artwork Management’s Digital Journey
Pharma artwork management has evolved through three major shifts, each addressing limitations the previous approach couldn’t solve. Manual, paper-based reviews were the standard for decades, with physical proofs and postal approvals that worked when product lines were simple.. This approach broke down as global markets expanded and regulatory complexity increased.
Digital desktop tools came next. PDFs, email markup, and local file servers improved efficiency, but teams still worked in silos with disconnected systems and manual handoffs that slowed approvals.
Today’s cloud-based platforms with AI capabilities represent the latest step forward. These systems provide centralized artwork libraries, real-time global collaboration, and automated verification for text, barcodes, and color accuracy. AI now handles content generation, intelligent approval routing, and quality control checks that catch errors human reviewers miss. Built with version control and audit trails that meet strict regulatory standards, these tools give teams more control over the artwork flow, from initial drafts to final approvals.
Understanding Critical Pharma Artwork Management Challenges
Pharmaceutical companies face challenges on every front, including shifting regulatory requirements, expanding product portfolios, and constant pressure to bring products to market faster. Global regulations don’t play by the same rules. Each region has its own set of formats, safety statements, and approval steps - and it’s up to pharma teams to keep up. Add in miscommunication, unclear roles, and messy workflows, and delays start stacking up fast.
That’s where a strong artwork management system makes a real difference. It keeps everyone aligned and working from the same set of accurate files, allowing teams to catch issues early and keep things moving without last-minute surprises.
Navigating Regulatory Compliance in Artwork Management
Regulatory bodies such as the FDA, EMA, MHRA, and Health Canada all have different expectations for packaging artwork. From black box warnings to layout formats, the details matter. Non-compliance consequences extend beyond recalls to include warning letters, consent decrees, fines, and facility restrictions that can halt operations.
Regulations change constantly, and it’s not always easy to keep up. Many teams rely on trusted consultants, regulatory bulletins, and internal reviews to stay ahead. The key is knowing how those changes affect your existing artwork, and having a process in place to respond quickly when they do.
An artwork management system supports compliance by applying country-specific templates, linking regulatory updates directly to affected content, and maintaining detailed audit trails. Pharmacovigilance teams rely on accurate, up-to-date safety info, especially when it comes to promotional materials. These systems help track safety changes, link them back to their source updates, and maintain a clear record of who approved what and when.
Building Quality Control in Artwork Management
Accuracy isn't optional in the pharmaceutical industry, which is why quality control begins with role-specific checks. Regulatory teams review label claims, medical teams verify content, and packaging experts confirm layout and print specifications.
Advanced proofreading technology supports each step through integrated quality management systems that compare text, graphics, barcodes and QR codes, and spelling against approved content. Connected to artwork management systems, these tools catch issues early and enable fast, documented action. Error prevention strategies track quality metrics to identify recurring mistake patterns, implement standardized review checklists, and use historical data from audit trails to improve processes over time.
Modern Technology for Artwork Management
Modern artwork management software transforms how pharmaceutical companies move content from draft to approval. Key capabilities include:
- Automated workflows with assigned roles and deadlines
- Electronic signatures compliant with 21 CFR Part 11 and Annex 11
- Integration with ERP, PLM, and regulatory systems
- Security elements including data protection encryption, role-based access management, and detailed activity logging
- Mobile capabilities for remote review and approval with full audit trail compliance
Automation opportunities span multiple areas: template-based compliance checking ensures regulatory elements are present, automated routing sends flagged items to appropriate reviewers, and intelligent text recognition identifies potential regulatory inconsistencies before human review begins. Modern platforms integrate these capabilities directly into existing artwork management tools, so quality checks happen where teams already work.
Streamlining Artwork Processes for Speed
When artwork processes grow organically, inefficiencies add up through duplicated reviews, missed deadlines, and version control mishaps. Mapping out how artwork actually flows reveals where these bottlenecks occur.
The fix is often simpler than expected. Parallel approvals replace sequential reviews, cross-functional collaboration happens through shared project dashboards and automated escalation procedures, and clear decision-making authority keeps teams aligned. Version control best practices include check-in/check-out functionality, complete revision histories, and rollback capabilities.
Successful change implementation requires engaging stakeholders and providing role-specific training to build confidence with new processes, plus phased implementation, clear communication about benefits, and early wins to build momentum across departments.
Smart Risk Management in Artwork Management Changes
Every artwork change carries risk since even minor modifications like revised dosage, new barcodes, or layout tweaks can affect supply chain, regulatory submissions, or patient instructions. Assessment methods include evaluating changes against manufacturing specifications, regulatory requirements, and related product lines. A risk-based approach prioritizes high-impact changes like dosage modifications for extensive review while speeding up lower-risk formatting updates.
Minimizing reprints and recalls caused by labeling issues requires catching errors before production through automated compliance checking and maintaining rapid response capabilities with version histories, change logs, and role-based alerts when something slips through.
Maximizing ROI in Artwork Management
The right artwork process saves time and money. Fewer errors mean lower printing costs, faster approvals lead to quicker launches, and less rework keeps teams out of fire-drill mode. When calculating ROI, don’t forget labor costs for coordinators and reviewers, opportunity costs from delayed launches, and hidden fees like rushed printing.
To work smarter, many companies automate routine checks, cut down on manual handoffs, and use scalable systems that handle growth. Some keep core tasks in-house but outsource specialized services like translation, matching skills to needs without overloading internal teams.
Global Artwork Management Made Simple
Global markets introduce new layers of complexity where labels must be translated, localized, and compliant with different rules, and mistakes here create real risk. Companies often choose between centralized models that provide brand consistency and cost efficiency through shared resources, or decentralized approaches that offer local market flexibility and faster decision-making. Most use a hybrid approach with centralized brand control and localized artwork execution. The right system makes this easier by managing region-specific templates and tracking updates across markets.
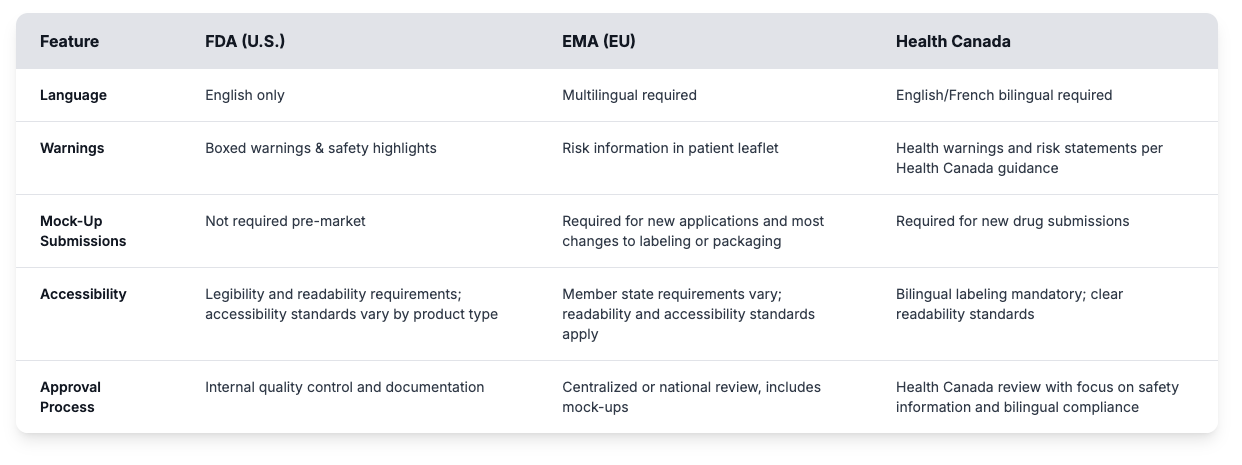
Understanding what each market needs, and having one system that keeps it all in sync, is how global teams move faster without risking compliance.
Sustainable Packaging in the Modern Era
Sustainability goals are becoming regulatory expectations, which means teams must now factor in recycling symbols, disposal instructions, and environmental claims without sacrificing compliance. In some markets, this includes mandatory on-pack recycling logos or carbon footprint disclosures. But the biggest sustainability impact comes from preventing waste before it happens.
Every labeling error that reaches production creates a cascade of environmental waste. Misprinted packages mean wasted materials, energy, and transportation. Recalls multiply this impact - product gets pulled from shelves, destroyed, and reprinted. A single batch recall can generate thousands of pounds of waste across packaging materials, finished products, and the carbon footprint of emergency reprints and redistributed shipments.
An artwork management system reduces this waste at the source by catching errors before production begins. Automated verification prevents the misprints that lead to recalls, while centralized templates ensure sustainability messaging stays consistent across markets. Version control reduces the risk of outdated or non-compliant packaging making it to production, eliminating the waste cycle that starts with a simple oversight.
The Future of Pharma Artwork Management
Cloud-based platforms continue expanding capabilities for real-time global collaboration while maintaining security and audit requirements pharmaceutical companies demand. These platforms cut down on IT overhead and handle updates automatically, so teams can focus on launching products rather than managing systems. As technology evolves in the life sciences industry, choosing the right solution that can support continuous innovation becomes just as important as the tools themselves.
AI applications are now delivering measurable results in pharmaceutical artwork management processes. Current AI capabilities include intelligent text recognition that flags potential regulatory inconsistencies, automated compliance checking against current requirements, and systems that identify recurring error patterns to reduce errors before they impact product launches. These technologies help ensure error free artworks while reducing the manual oversight that often creates bottlenecks in the process.
Predictive analytics is gaining traction in compliance and quality management, especially as teams look to stay ahead of regulatory changes. These tools help teams spot shifts in regulatory focus and uncover patterns in quality data, giving them a better chance to act early and adjust artwork processes before small issues turn into larger compliance challenges that could delay successful product launches.
As these technologies mature, the most successful pharmaceutical companies are those implementing comprehensive artwork management systems that combine human expertise with intelligent automation, making sure every approval meets both accuracy and compliance requirements.
Choosing Your Ideal Artwork Management Partner
The right partner knows your industry, fits into your systems, and has delivered results for teams like yours. They understand the pressure of working in regulated spaces, and how to keep things moving without sacrificing accuracy.
Before you commit, ask questions that reveal how they really work:
- Do they integrate with your existing regulatory and enterprise systems?
- Can they support your regulatory compliance needs?
- How do they handle change management and version control?
- What does onboarding and support look like?
Building effective partnerships requires shared performance metrics, regular business reviews, and clear communication protocols. Managing outsourced artwork relationships effectively means balancing oversight with operational flexibility - maintain sufficient control for quality and compliance while allowing vendors the autonomy needed for efficient service delivery. This includes establishing escalation procedures that address issues quickly without micromanaging day-to-day operations.
Speed becomes critical when evaluating potential partners. Artwork delays directly impact timelines, so look for vendors who demonstrate how their processes actually reduce revision rounds, rather than just manage them. The best partners prevent delays by handling upfront planning and stakeholder coordination effectively. They use quality checks that catch issues early, before problems turn into multiple revision cycles that can derail launch timelines.The right partner doesn’t just offer software. They bring experience, training, and a track record of helping teams reduce errors and move faster.
Real Success Stories Artwork Management Transformations
Two pharmaceutical companies faced different artwork challenges but found similar solutions through automated quality inspection.
Case Study 1: iNova Pharmaceutical - Bringing Control Back In-House
iNova Pharmaceuticals operates across 20+ countries with products ranging from weight management to dermatology. In 2017, they moved their artwork processes in-house after working with an external studio that offered limited control. GlobalVision’s software helped them take ownership of quality assurance, improving their quality revisions and increasing their speed-to-market for new products. See how iNova transformed their artwork process.
Case Study 2: Biogen - Eliminating Costly Delays
Biogen’s legacy systems created delays and million-dollar losses where even minor changes meant rebuilding processes from scratch. After implementing GlobalVision in 2019, they saw over $1.2 million in annual savings, a 12% acceleration in artwork cycle time, and 100% reliability. The system reduced delays across artwork teams, regulatory, quality partners, and suppliers, helping them get medicines to market faster. Learn more about Biogen’s results.
These examples show that whether you’re bringing processes in-house or replacing outdated systems, the right quality inspection platform delivers measurable improvements in speed and accuracy.
Your Implementation Roadmap for Improved Artwork Management
Start with a clear baseline assessment of current state and gap analysis. Where are errors happening? Which steps take the longest? Who owns what? Map your existing artwork development process to identify inefficiencies and set benchmarks for improvement.

Step-by-step transformation begins with pilot projects using a single product line where you can set clear success metrics and run parallel processes until teams master new workflows. Change management becomes critical - involve key departments early, communicate benefits clearly, and address resistance through role-specific training that builds confidence with new processes.
Track results early to demonstrate fewer errors, faster approvals, and stronger audit readiness. Those wins help build momentum across the rest of your organization.
Artwork mistakes delay launches, invite audits, and risk patient safety. But with a system designed for the pharmaceutical industry, complete with version control, quality checks, and built-in efficiency, your artwork process becomes a strength, not a scramble.
See for yourself how GlobalVision’s Verify eliminates errors in your pharmaceutical artwork process.



