Quality control software forms the backbone of compliance in regulated industries, yet many teams struggle with outdated tools that can't keep pace with modern packaging complexity. Comparing GlobalVision’s Verify against Schlafender Hase’s TVT reveals significant differences beyond surface features. This analysis focuses on what truly matters: accuracy, compliance, and speed. These factors are non-negotiable for pharma, medical device, and life sciences teams working with increasingly tight launch timelines.
Quick comparison: GlobalVision vs Schlafender Hase
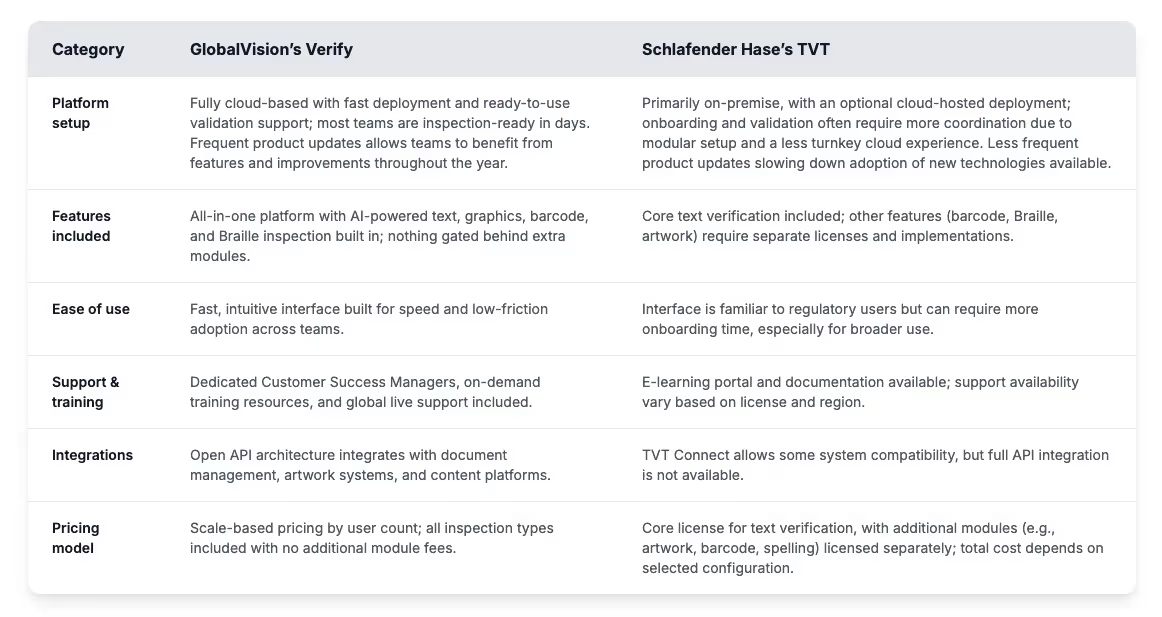
At a glance: What both platforms offer
GlobalVision’s Verify and Schlafender Hase’s TVT excel at one critical function: preventing errors in regulated content from reaching the market. Their core technology uses Unicode character-by-character text verification to catch discrepancies human eyes miss. Both platforms generate comprehensive inspection reports for pharmaceutical labelling compliance that satisfy FDA and EMA regulatory documentation requirements. Teams transitioning from manual proofreading to digital verification will find that either solution dramatically improves accuracy while reducing review times.
On technical fundamentals, the platforms share common ground. Each supports the standard formats required in regulatory workflows, including PDF, Word (including QRD format), Excel,XML, CSV, and ZIP files containing SPL documents. They also provide validation documentation that meets regulatory compliance environment requirements. Their real differences are in platform architecture, user experience design, and their approach to solving complex packaging challenges. These differences determine which solution works best for different verification needs.
Where Schlafender Hase excels
TVT has built a strong reputation through fundamental text verification capabilities that pharma teams trust, particularly in European markets. Regulatory professionals rely on its character-matching algorithms to detect even the most subtle text changes when reviewing patient information leaflets, instructions for use, and submission documents. The platform provides a stable, predictable environment for teams who have already validated TVT in their compliance workflows, with audit trail capabilities that fulfill Good Manufacturing Practice requirements through exportable documentation accepted by major regulatory bodies.
The platform particularly shines when handling complex multilingual text documents and submission materials, prioritizing content accuracy over visual presentation. TVT offers specialized features tailored to pharmaceutical documentation review that many regulatory affairs departments value. This focused approach works well within established pharma validation processes, allowing teams to maintain continuity in their compliance documentation.
Organizations with dedicated verification specialists often find TVT aligns well with their existing regulatory workflows. These specialized teams typically have resources allocated specifically to text verification tasks and follow structured review processes. Since these teams concentrate on regulatory documentation rather than cross-departmental packaging review, TVT's text-centric approach matches their primary verification requirements.
Where GlobalVision stands out
GlobalVision’s Verify takes a fundamentally different approach by unifying all inspection types in one platform. Text verification, visual inspection, barcode grading, and Braille translation work together effortlessly, eliminating the procurement headaches and validation burdens of juggling separate modules. This unified design tackles a common challenge for regulated teams: spreading verification responsibilities beyond specialized regulatory staff to packaging, marketing, and quality teams.
The platform's intuitive interface allows occasional users to become productive without extensive training, pushing quality checks into earlier stages of content development. When tight launch deadlines collide with strict compliance requirements, Verify delivers faster implementation through cloud-based deployment. Teams are ready for inspection tasks within days, not weeks, with validation packages built for quick qualification that deliver measurable ROI from the first verification projects.
Integration capabilities further strengthen Verify's position through API technology that connects verification to document management platforms and artwork approval systems. This creates a continuous quality thread throughout the regulated content lifecycle rather than isolated checkpoints. For organizations scaling verification across departments, regions, or product lines, the platform's architecture eliminates bottlenecks while its comprehensive multilingual capabilities (including 44 Braille languages) address international labeling requirements without additional module investments.
Feature-by-feature breakdown
Both platforms verify text with high precision, but their approaches create distinctly different user experiences and results in real-world settings.
Accuracy and speed: Verify unifies text, graphics, barcode, and Braille inspection in a single interface. Their cloud platform processes multi-page documents rapidly, cutting verification time dramatically compared to manual methods. TVT splits these capabilities into separate modules. Each works well on its own, but requires users to juggle multiple workflows for complete package verification, adding extra steps and training time to the process.
Visual inspection: The biggest difference between platforms is when verifying text and graphics. Verify includes visual inspection as standard, with pixel-by-pixel comparison that catches artwork differences immediately. Their Graphics Comparison technology adjusts sensitivity to detect critical details while filtering out noise. TVT focuses on text in its core platform, offering visual inspection only through the separate TVT Artwork add-on. While this module provides features like high-level zoom and layer selection, it requires additional purchase and implementation beyond the base system. Visual inspection matters particularly for medical device and pharma teams, where packaging errors can trigger recalls. Regulatory symbols, warning icons, and dosage charts require pixel-level accuracy for compliance. Verify's approach recognizes that effective packaging verification must handle these visual elements alongside text, not as separate processes.
Regulatory compliance: Both platforms deliver comprehensive audit trails that track user actions, document inspection results, and support 21 CFR Part 11 and Annex 11 compliance (FDA's electronic records requirements for regulated industries). Both generate reports accepted by global regulatory bodies, but Verify adds ISO 27001 certification for its cloud environment on AWS. Their reporting unifies all inspection types in submission-ready formats. TVT's compliance documentation starts with text verification and expands when using additional modules, giving teams with established TVT validation procedures a familiar path forward.
Support and onboarding: Implementation speed varies significantly between platforms. Getting started with Verify takes less effort from internal teams. Deployment happens quickly through cloud access, and validation kits come ready to use. Regulatory, labeling, and quality teams can begin working in the system within days. It’s a single qualification process across text, barcode and Braille QA, artwork, and packaging review. TVT requires more coordination. Most teams install and validate modules separately, which adds work for QA leads and slows down regulated content lifecycle workflows. Cross-functional usage becomes harder when different groups are stuck on different tools.

Integration and scalability: As verification becomes integral to broader regulated content workflows, connectivity matters more. Regulated content workflows don’t run in silos. Teams use document control systems, artwork tools, and promotional review platforms together. Verify was designed to connect across all of them. Its API-based integration supports audit-ready processes without revalidating each connection, reducing friction between departments and keeping verification tied to the regulated content lifecycle. TVT’s integration approach, centered on TVT Connect, supports basic file handling but lacks the flexibility of full API access. Larger organizations face issues when expanding across product lines or regions. TVT’s on-prem QA tools also depend on older deployment methods like CITRIX, which adds overhead for IT and limits access control. There’s no modern SSO support, which matters when scaling enterprise quality management systems.
Pricing, onboarding, and long-term ROI
Modular software may seem cost-effective early on. But that changes when requirements expand. TVT’s model starts with core text verification. Each added module - visual inspection, barcode inspection, Braille, or spelling - carries separate pricing, setup, and validation. Teams often discover these gaps mid-project, leading to delays and new budget approvals. Verify uses a single-platform approach. Visual inspection vs text verification isn’t a choice, it’s built in. Pricing reflects the size of your organization, not the number of inspection types, keeping costs predictable as your QA scope grows.
Timelines significantly impact total cost of ownership. Verify deployments typically complete within days, with full validation in weeks. Their cloud-based architecture eliminates infrastructure setup and costly IT involvement. TVT implementations generally take longer, particularly when installing multiple modules in on-premise environments. Each module introduces separate validation requirements, extending time-to-value for complete package verification. The difference becomes especially pronounced for organizations implementing comprehensive inspection across text, artwork, barcodes, and Braille, where Verify's unified approach simplifies validation documentation.
The vendors take distinctly different approaches to customer success that mirror their platform designs. Verify provides implementation playbooks and ongoing process improvements designed to accelerate value realization across departments, while TVT focuses on technical documentation and application-specific support for specialized users. Both companies demonstrate commitment to regulated industries, but Verify's approach emphasizes cross-functional usage while TVT targets dedicated regulatory specialists. This difference affects how quickly teams across packaging, quality, and regulatory departments can become productive users.
Which one is right for you?
Your verification needs, team structure, and growth plans should guide your platform choice. For teams that focus only on text-based regulatory documentation, such as product information documents, patient leaflets, or submission forms, Schlafender Hase’s TVT may meet current needs. It’s familiar to many regulatory professionals in pharma and supports pharma labeling compliance with character-by-character text verification. However, most labeling-heavy industries now manage much more than text. Medical device, CPG, and global life sciences organizations need tools that handle the full scope of error detection in regulated workflows, including visual inspection, barcode and Braille QA, and version control across departments. GlobalVision Verify was built with that in mind. It supports multilingual packaging QA, connects to your broader content ecosystem, and keeps pace with FDA and EMA audit support requirements, without asking teams to switch tools midstream.
The situation looks different for teams managing more than just regulatory documentation. In pharma, as well as in medical device and CPG, packaging inspection now spans multiple content types and compliance checkpoints.Verify's unified approach eliminates the fragmentation of using separate tools for text, artwork, barcodes, and Braille verification, making it a strong fit for not only traditional pharma workflows, but also for teams navigating complex, multi-product labeling environments.
Implementation timelines and future growth also shape the decision. Teams needing fast quality improvements typically see quicker results with Verify's rapid deployment and pre-validated packages. While TVT's modular structure allows staged rollouts, the total cost often exceeds initial estimates as verification needs expand. Companies growing into new markets or adding product lines find Verify's cloud platform scales more easily across departments. Its built-in multilingual packaging QA and workflow integration capabilities support organizations expanding beyond traditional pharmaceutical boundaries into other regulated industries where comprehensive verification becomes increasingly critical.




