Medical devices are only as reliable as the packaging that protects them. Healthcare technology advances at breakneck speed, but the real innovation happens behind the scenes - in the packaging solutions keeping critical tools safe and sterile when lives depend on them.
Overview of the Global Medical Device Packaging Market
The medical device packaging market was worth USD 33.5 billion in 2023. And it isn’t slowing down. Projections show it hitting USD 54.1 billion by 2029, growing at 6.3% a year. Behind those numbers are two major drivers: aging populations that increase demand for medical devices, and advanced medical technologies that need specialized protection.
Specialized packaging solutions have become critical for medical devices because standard packaging simply can't handle the demands. Medical equipment requires protection that maintains sterility and product integrity throughout complex global supply chains, along with clear regulatory compliance documentation every step of the way. Protecting medical devices in this way is central to regulatory compliance and patient safety.
Growth in the medical device packaging market comes from a mix of forces now reshaping how devices are protected and delivered:
- Demographic shifts: Longer lifespans are putting more medical devices into circulation.
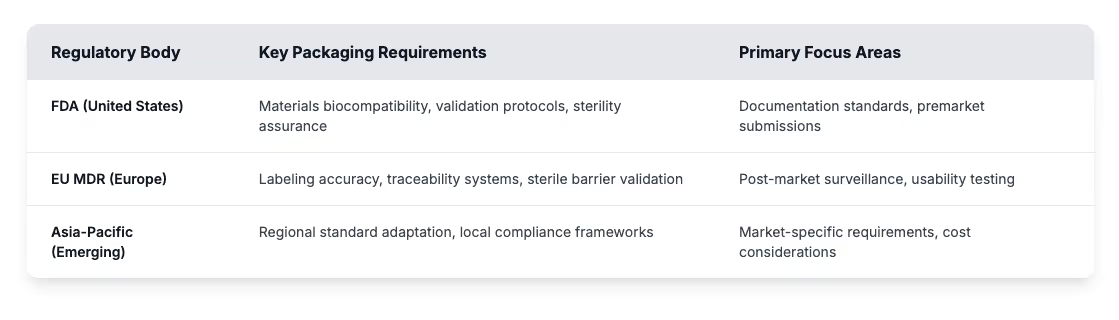
- Tighter regulations: FDA and EU standards are raising the bar for safety and compliance.
- Device complexity: Smart devices and implantables now demand packaging that maintains sterility and data integrity while surviving global supply chains.
- Innovation in materials and systems: Advanced materials, smart packaging technologies, and automated production systems are being used to strengthen reliability and support compliance.
Regional dynamics shape growth in different ways. North America holds the largest market share, backed by advanced healthcare infrastructure and high adoption of medical technology. Asia Pacific is growing faster than other regions as China, India, and Southeast Asian countries expand healthcare access, while Europe balances steady demand with a regulatory focus and sustainability leadership that’s reshaping global packaging expectations.
Market Dynamics and Key Growth Drivers
As medical devices advance, packaging has to keep pace. Implants now require electromagnetic shielding as part of their packaging design. Diagnostic equipment raises a different issue, with data integrity needing protection across complex supply chains. Many newer medical technologies add another layer of difficulty by requiring tightly controlled environments from production all the way to point of use. Every breakthrough in medical technology creates new packaging challenges that manufacturers have to solve.
Demand is rising on every front. Aging populations in developed countries need more medical interventions, from routine diagnostics to complex surgeries. Emerging markets are still developing healthcare infrastructure, which is driving demand for cost-effective packaging solutions that maintain patient safety. At the same time, regulatory expectations continue to intensify. FDA rules, EU MDR requirements, and evolving Asia-Pacific frameworks all place new emphasis on protection, labeling, and traceability across the healthcare industry. These stricter requirements actually drive innovation in materials science along with smart packaging technologies and improved supply chain transparency.
Put together, these forces sustain market growth. Healthcare systems globally invest heavily in advanced medical devices while chronic diseases drive demand for long-term treatment solutions. The result? A continuous demand for packaging solutions that can keep pace with healthcare’s rapid evolution.
Material Innovations Transforming Medical Device Packaging
Plastics represent the majority of packaging materials across the industry for good reason. They're versatile and cost-effective while handling sterilization processes that would destroy other materials. The lightweight nature matters too when you're shipping medical devices globally.
The industry keeps moving, though. Sustainable packaging solutions have become a focus as healthcare organizations balance environmental goals with patient safety. Companies such as Sonoco Products Company are driving innovation in this area, developing biodegradable materials that maintain sterile barriers alongside recyclable plastics for medical use. Other manufacturers are cutting packaging volume to reduce waste. But sustainability can't compromise protection through months of storage and international shipping. That's why material innovation matters more than simple reduction - swapping materials without redesigning the system rarely works. Choosing the right packaging materials gets complicated fast depending on what you're protecting:
- Implantable devices often use materials that are safe for long-term human contact and can endure multiple sterilization cycles.
- Diagnostic equipment packaging must guard against electromagnetic interference and physical shocks during transport.
- Surgical instruments require materials resilient enough for repeated sterilization without barrier breakdown.
- Connected devices bring unique needs, including protection for sensitive electronics and assurance of data integrity.
Smart materials are changing the game too. These advanced packaging technologies monitor temperature and humidity along with other environmental factors that can make or break device safety. The packaging materials provide real-time data that helps healthcare providers verify product integrity and optimize inventory management throughout the supply chain.
Product Types and Specialized Packaging Solutions
Bags and pouches represent the most common packaging choice in the device packaging market. They are inexpensive to produce and simple to scale, while also offering the flexibility needed for varied device shapes. In practice, this format is used for surgical instruments in operating rooms, diagnostic equipment in labs, and sensitive electronics handled in healthcare facilities. Not every device, however, can be packaged in this way. When greater protection is required, rigid formats come into play. Primary packaging provides direct contact protection for the device itself, while tertiary packaging covers the broader logistics layer, bundling shipments for distribution. Here’s how the most common formats compare:

Costs also vary significantly by format. Standard pouches and bags offer the lowest unit cost, whereas custom thermoformed trays or specialized containers carry a higher price but deliver superior protection for high-value equipment. For many medical device manufacturers, the calculation goes beyond purchase price. Packaging that prevents transit damage can also simplify warehouse handling and shorten surgical setup, creating a stronger return on investment. As a result, total cost of ownership is becoming a more common measure than unit pricing alone.
Application-Specific Requirements Across Medical Device Sectors
Different medical device sectors create completely different packaging challenges. Monitoring and diagnostic equipment contains sensitive electronics that need protection from shock and vibration, plus electromagnetic interference throughout global shipping. Anti-static materials matter here, along with cushioning systems and environmental controls. A single impact during transport can throw off calibration accuracy for months.
Sterile packaging for implantable devices represents the most demanding application in the medical device packaging industry. For surgical instruments, packaging materials must withstand repeated sterilization without barrier breakdown. Connected medical devices present a different challenge: they require shielding for sensitive electronics and assurance of data integrity, factors that were rarely part of packaging design until recently.
Advances in medical technology are also raising the bar for packaging. Surgical instruments call for materials that can handle repeated sterilization without barrier breakdown, while connected medical devices now introduce requirements for electronic shielding and data integrity that were not part of packaging design in the past.
Sterility Maintenance: The Foundation of Medical Device Packaging
Sterility is what separates successful medical device packaging from failure in the field. Packaging has to withstand sterilization by gamma radiation, ethylene oxide, or steam while keeping its barrier intact. The challenge is letting sterilization in without letting contamination through over the device’s entire lifecycle. A single breach in sterility can have direct consequences for patient safety, which is why validation begins long before production.
Quality systems in sterile packaging focus on accuracy at every stage. Medical device packaging manufacturers use validation protocols to confirm performance under real conditions, from sealing through to final use. Because a single failed batch can halt production and cause recalls, testing remains essential for both patient safety and regulatory compliance. Building sterility validation into early design stages also helps reduce rework and keeps development timelines on track.
Regulatory bodies keep raising standards for validation and labeling alongside more complex traceability requirements. As medical device technologies advance, compliance expectations evolve with them. Packaging manufacturers are expected to stay ahead of regulatory shifts and guide customers through new requirements.

Regional Market Analysis: Opportunities Across Global Healthcare Systems
North America accounted for the largest share of the medical device packaging market in 2023, supported by advanced healthcare infrastructure and high adoption of medical technology. Strong research partnerships and direct clinical feedback also shape how packaging solutions evolve in the region. Europe shows similar maturity but stands out for its stronger focus on sustainability and circular economy principles. The EU MDR adds regulatory complexity, but packaging manufacturers that adapt quickly often find an advantage in a market that values compliance as highly as performance.
Asia Pacific presents the strongest growth opportunities in the global medical device packaging sector. Healthcare access is expanding in India, China, and Southeast Asia, and the pace of infrastructure development in these regions is ahead of other markets.
Emerging Market Opportunities
Developing economies show massive expansion potential as governments invest heavily in healthcare infrastructure while growing middle classes demand better medical care. In emerging markets, packaging manufacturers often need different approaches than in developed regions. Regional manufacturing hubs are reshaping supply chains as countries expand medical device production, which increases demand for local packaging expertise that supports both domestic use and export.
Localization strategies are central here. Providers in these markets face several pressures: regulations that differ from FDA and EU standards, cultural expectations in healthcare delivery, and tighter economic limits that make cost-effective solutions essential. Companies that balance these regional factors with global quality standards are the ones able to sustain growth over time.
Technological Advancements Revolutionizing Medical Packaging
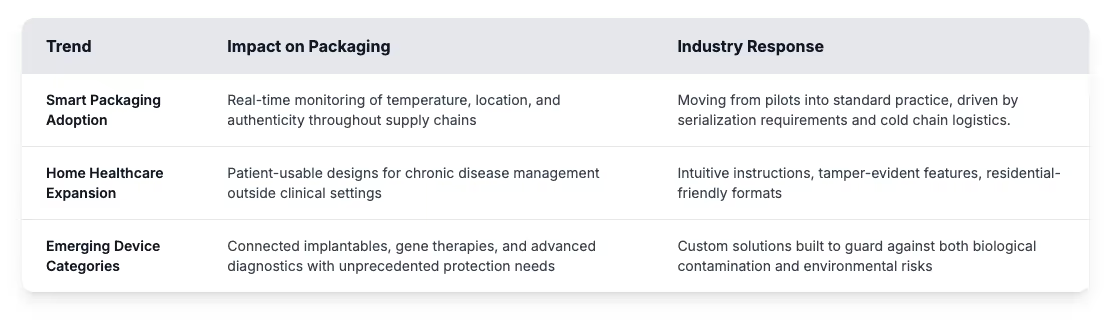
Artificial intelligence is applied throughout medical device packaging, from early design through production. AI applications draw on material performance data to identify potential failure points, giving packaging teams insight to refine designs before physical prototypes are built. Machine learning also plays a role in quality control, where inspection systems can pick up subtle defects that often escape human review, while reducing the false positives that slow production lines.
Packaging itself is becoming smarter. Beyond design and inspection, today’s systems provide active monitoring - from tracking conditions during transport to verifying authenticity. Many also connect directly with healthcare information systems across the product lifecycle. Sensors, RFID tags, and other connected tools give packaging a larger role in traceability and in protecting patient safety. In parallel, advances in materials are adding protective features, with some designed to change color when exposed to damaging conditions, and others developed to maintain humidity levels or show tamper evidence.
On the production floor, automation is what ties these technologies together. Industry leaders are putting automation at the center of packaging operations. Automated lines cut down on human error and raise throughput while recording every step of the process. Automation is how manufacturers keep precision intact as production scales.
Regulatory Landscape: Navigating Complex Compliance Requirements
Regulatory demands shape everything about medical device packaging development. FDA standards, EU MDR requirements, and Asia-Pacific frameworks are all pushing packaging toward stronger materials and stricter controls on labeling and traceability across the healthcare industry. These expectations influence packaging decisions before materials get selected and continue through final validation testing.
Unique Device Identification (UDI) systems assign each medical device a unique code to support traceability and patient safety. FDA and international regulators now require packaging that accommodates barcodes or RFID tags tracking devices through their entire lifecycle. Medical device packaging manufacturers now need to add tracking features into labels that already carry product information, regulatory symbols, and safety warnings, and still protect package integrity and usability. Requirements aren’t consistent worldwide - markets ask for different details in different formats, and UDI timelines vary by region, which makes implementation complex.
Smart packaging manufacturers collaborate with device companies during early product development rather than waiting for finalized designs. This approach surfaces regulatory requirements when changes cost less and move faster, preventing the expensive redesigns that blow up timelines when packaging gaps show up during validation testing.
Supply Chain Considerations in a Connected World
Distribution channels have evolved significantly as the medical device industry has globalized. Direct-to-hospital shipments compete with complex distributor networks that add efficiency but also add touchpoints where something can go wrong. Emergency supply chains for critical devices demand packaging that maintains integrity under accelerated timelines and less-than-ideal handling. Protective packaging solutions have to work across air and ocean transport so manufacturers don’t need separate systems for each distribution method. The challenge grows when raw material sourcing is added, since the specialized materials required for medical devices often come from a small group of suppliers.
Supply chain disruptions in recent years exposed this concentration risk. When transport slowed or raw materials were in short supply, some medical device manufacturers struggled to get critical equipment to patients. Packaging manufacturers have developed systems that extend shelf life without refrigeration and reduce the storage space needed in constrained warehouses. These same systems also maintain sterility when packages sit in non-climate-controlled environments for longer periods. Manufacturers are diversifying their supplier networks geographically and investing in material science research to identify alternatives to scarce resources, reducing dependence on single sources while still meeting the performance standards required for regulatory compliance.
The real test of medical device packaging plays out in conditions nobody plans for. Packages shipped through tropical heat and stored in basic warehouses by personnel with minimal training must protect devices as effectively as those moving through premium logistics networks. Healthcare providers in emerging markets depend on packaging solutions that compensate for infrastructure gaps.
Competitive Landscape: Innovation Through Partnership
In the medical device packaging market, multinational companies such as Amcor, and DuPont operate through extensive manufacturing networks and materials expertise. Specialized firms - including Nelipak, SteriPack, and Oliver Healthcare Packaging - differentiate themselves through technical precision and faster customization. West Pharmaceutical Services carved out its niche in pharmaceutical packaging systems that extend into medical devices, while Sonoco Products Company leads in paperboard solutions and sustainable materials that address growing environmental pressures.
Consolidation reshaped the landscape through 2024 and into 2025. Major acquisitions showed how scale and sustainability now drive competitive strategy in the medical packaging market. Mid-sized players similarly merged to reach the scale global medical device contracts demand, with much of this activity targeting Asia Pacific markets where medical device manufacturing keeps accelerating. Companies without a manufacturing base in key growth regions struggle to stay competitive. To fill that gap, device manufacturers look for packaging partners that can support global supply chains and meet strict quality and delivery standards. In this environment, packaging providers usually set themselves apart in three ways:
- Material innovators are known for proprietary technologies, including high-barrier films, sterilization-compatible polymers, and sustainable alternatives.
- Scale players operate through broad manufacturing footprints and vertical integration, giving medical device companies consistent support across regions and packaging formats.
- Specialists carve out niches in thermoformed trays for surgical kits, flexible pouches for diagnostics, or custom solutions for implantable devices, where focused expertise commands premium pricing.
Strategic partnerships look different today. Packaging partners now contribute to design and sterilization validation while also providing the regulatory documentation needed for compliance and faster approvals. This support is most critical for emerging medical device companies and firms entering new markets, where packaging expertise can prevent costly redesigns and delays. The strongest partners understand FDA and EU MDR requirements and adapt manufacturing schedules to device development speed rather than locking companies into rigid production cycles. Regional manufacturers also maintain influence after consolidation by meeting local regulatory requirements and competing on cost and service. Their presence gives device manufacturers leverage in negotiations and helps drive the packaging innovation that brings life-saving technologies to patients faster.
Sustainability Trends Balancing Safety and Environmental Responsibility
Single-use plastics still dominate medical device packaging, able to maintain sterile barriers during harsh sterilization and remain stable over long shelf lives. Alongside plastics, materials like foil laminates and PETG thermoforms are widely used because they protect against contamination during gamma radiation and ethylene oxide sterilization - conditions that can compromise many biodegradable alternatives. The drawback is that the same materials safeguarding patients also produce waste, most of which is ultimately incinerated.
New advances in material science are creating packaging that maintains sterility while lowering environmental impact. The result is a shift away from plastics alone, with more sustainable alternatives beginning to take hold:
- Mono-material systems use the same polymer family for both tray and lid, allowing them to withstand gamma sterilization and the mechanical stress of heavy devices while remaining recyclable in facilities with the right infrastructure.
- Traditional laminates relied on multi-layer builds that blocked recycling streams entirely.
- Newer designs simplify recycling by using identical top and bottom webs, so entire packages can be processed without disrupting hospital workflows.
Where sterility requirements allow, paper-based options are emerging. Medical-grade kraft papers from sustainably managed forests maintain sterile barriers over extended periods while working with standard sterilization methods. Once considered dependent on plastics, categories like surgical supplies and diagnostic equipment are now shifting toward paper-based packaging. Plastic remains essential for implantables and complex electronics, but moving suitable product lines into paper has already cut plastic use across high-volume segments.
One way to improve sustainability is by reducing the amount of material used. Waste drops when blister packs are right-sized and outer layers are trimmed, especially for devices designed to need less protective packaging. More compact packages also lower transport emissions and reduce storage needs across the supply chain. Manufacturers willing to rethink their approach have cut packaging volume substantially through design optimization, with the impact showing up across the entire cost structure.
The EU’s proposed Packaging and Packaging Waste Regulation directs manufacturers toward recyclable designs and simpler material use. New traceability rules are also making it easier to include recycled content in non-sterile packaging components. Together, these shifts are changing business priorities as medical device companies begin to weigh environmental performance alongside sterility assurance and regulatory compliance.
Collaboration across the value chain is becoming essential. Packaging manufacturers are developing materials that can handle both sterilization and recycling. Medical device companies are reducing packaging needs through product redesign. Healthcare facilities are adding systems that separate recyclable materials from contaminated waste. None of these pieces work in isolation, but together they're building infrastructure for circular healthcare packaging that protects both patients and the planet.
Future Outlook: Innovation Driving Market Expansion
The medical device packaging market is projected to keep expanding through 2030, with growth outpacing much of the broader manufacturing sector. What’s driving this isn’t just demographics - it’s deeper shifts in how medical devices reach patients, where they’re used, and what packaging has to deliver beyond basic protection.
North America is seeing steadier growth, shaped by regulatory modernization and new sustainability mandates. In Europe, circular economy initiatives are adding pressure on packaging manufacturers to innovate within stricter environmental requirements. Growth in Latin America and Southeast Asia appears to be accelerating as expanding middle-class populations gain greater access to healthcare.
Three Major Trends Reshaping Packaging Requirements
Sustainability is no longer secondary in supplier selection. Medical device companies now weigh environmental performance alongside quality and compliance when choosing packaging partners. Their evaluation tends to center on three areas:
- Regulatory expertise delivered at speed
- Geographic reach across mature and emerging markets
- Quality systems that scale globally
The outlook through 2030 shows packaging moving from a support function to a central driver of how medical devices reach patients. The next stage of growth depends on how well packaging supports both innovation and accountability across the device lifecycle.
GlobalVision’s Verify gives packaging teams confidence that every label and file meets the accuracy regulators expect and patients depend on.