For quality professionals, choosing between GlobalVision’s Verify and InformaIT is about more than software. It is a decision tied to consumer safety and non-compliance risks, regulatory expectations, and day-to-day quality performance.
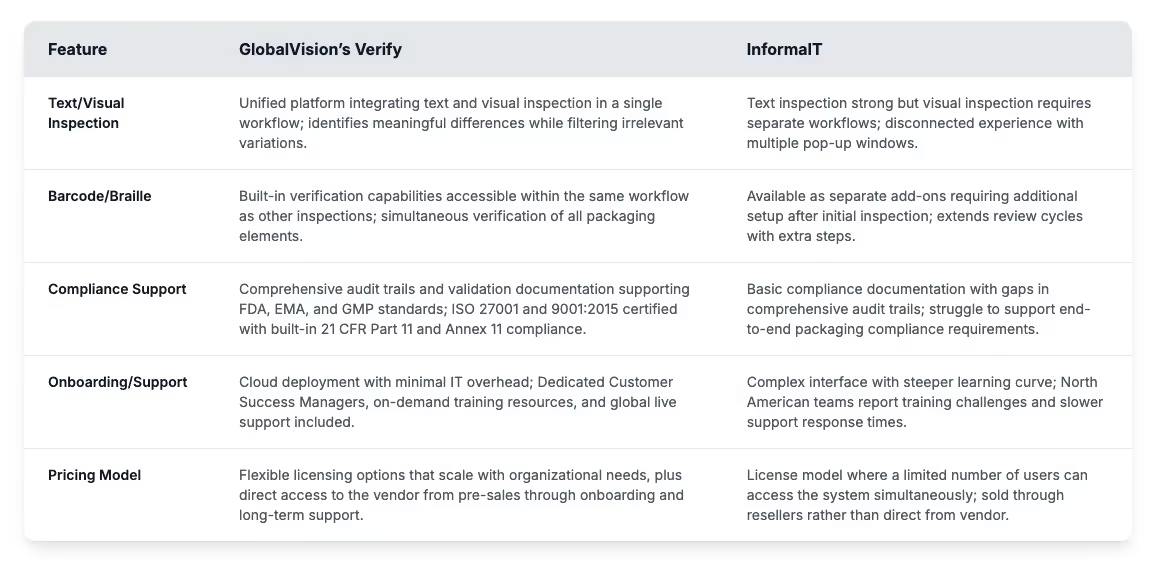
Quick comparison: GlobalVision vs InformaIT
Where both platforms align
Quality professionals in pharmaceuticals and life sciences face a fundamental challenge: catch errors before they become costly problems. Verify and InformaIT tackle this head-on, providing specialized verification tools that identify discrepancies in regulated documentation and packaging with precision that generic comparison tools simply can't match.
Both platforms excel at identifying issues in regulated documents before they become compliance risks. This capability separates purpose-built quality control tools from general office software when working with critical materials like package inserts and regulatory submissions.
These platforms approach verification with an understanding that not all differences carry the same weight. Even small changes can lead to serious regulatory consequences in pharmaceutical and life science documentation. Both solutions are built to support that level of precision. This shared focus on preventing errors while meeting quality standards provides the foundation from which both tools operate, though their approaches to achieving these goals differ significantly.
Where InformaIT performs well
InformaIT proofreading works well for regulatory teams that focus on text. Its comparison engine checks documents line by line, helping users catch small changes in submissions and package inserts. For teams that only need to review content, not layout or graphics, InformaIT can meet those needs.
The platform’s lightweight footprint and focused approach to text verification provides clear value for organizations with straightforward quality control needs. When teams work mostly with text-heavy content and have limited visual inspection requirements, InformaIT delivers core functionality without the complexity of broader platforms.
Some regulatory teams, especially in parts of Europe, continue to use InformaIT to check fine text changes. For teams with steady review routines, long-term use of the platform adds a sense of stability.
Teams with standardized procedures built around text-focused verification may hesitate to change platforms, particularly when their quality efforts remain centered on documents. But as packaging becomes more complex and quality checks expand beyond text, many teams run into limitations with this approach.
Where GlobalVision leads
Verify integrates text, graphics, barcode, and Braille inspections into one system, unlike InformaIT, which requires separate tools and workflows for non-text elements. This eliminates the inefficiencies of switching between tools and supports content lifecycle QA at scale.
It’s purpose-built for pharma, medical devices, and CPG teams who must ensure multilingual packaging accuracy, FDA/EMA compliance, and audit-ready reporting across diverse assets. For pharma in particular, Verify supports specialized formats and regulatory workflows. Teams can inspect SPL (ZIPs with XML and JPEGs), run QRD template checks automatically, and review Braille content against the Marburg Medium standard used in EU packaging. The platform also includes continuously updated medical dictionaries to help reviewers flag terms and avoid critical errors in labeling.
Verify helps teams focus on the changes that matter by ignoring the ones that don’t. Its visual inspection tools and GMP compliance features meet high regulatory standards. Integrated audit trails, validation packages, and ISO certifications (27001 and 9001:2015) deliver document control and support audit readiness.
The cloud-based architecture enables powerful scaling across teams and content types. Verify creates continuous quality assurance throughout the content lifecycle by connecting verification directly to artwork management platforms and prepress quality tools.
GlobalVision pairs the Verify platform with hands-on onboarding and long-term support. Teams get guided training, access to an on-demand learning management system, and a dedicated customer success team that understands regulated workflows. This approach helps teams build confidence quickly and get full value from Verify across departments.
This approach helps distributed teams implement consistent standards across locations and product lines, making Verify particularly valuable for organizations managing diverse content types or expanding into new markets. Teams using Verify catch errors earlier, move through reviews faster, and turn compliance into a competitive advantage.

Feature comparison
InformaIT and Verify both address quality control needs for regulated industries, but their approaches create distinctly different experiences for teams working with complex packaging and documentation requirements. The differences between the platforms show up clearly in areas that affect how teams verify content. These include visual inspection, compliance, and how well the tools fit into daily workflows. Over time, those differences shape how teams find and fix errors.
Visual inspection capabilities: InformaIT offers text comparison but struggles with comprehensive packaging inspection. It requires teams to move between separate tools to check graphics, barcodes and layout, slowing the review process. Verify brings everything into one platform, so teams can inspect text, artwork, barcodes, and Braille in a single pass.
Automated error detection: The character-by-character verification in InformaIT flags every discrepancy regardless of significance, often leading to numerous false positives requiring manual review. This becomes especially challenging with multilingual content. Verify flags the changes that matter and filters out the ones that don’t. This helps quality teams focus on real issues instead of losing time on noise.
Regulatory compliance: As documentation requirements become more complex, InformaIT’s text-only approach makes it harder to meet full packaging compliance. Visual checks often require extra steps or additional tools. Verify was built to support full regulatory workflows, with audit trails, validation files, and security features aligning with FDA, EMA, and GMP expectations. It includes built-in support for 21 CFR Part 11 compliance.
Barcode/Braille verification: InformaIT handles barcode and Braille checks through separate steps or add-ons, which can slow reviews and create gaps. Verify includes both checks as part of its main system. Teams can review every packaging element in one workflow, reducing the chance of missing something important.
Platform usability: InformaIT uses multiple pop-up windows to show results, and reviewers must click through extra screens to see changes. That setup can slow users down, especially if they’re not in the tool daily. Verify keeps everything in one place. Its interface is built for daily work, helping teams focus on reviews instead of figuring out how to move through the system.
Integration capabilities: Quality teams now need tools that connect to the rest of their content systems. InformaIT doesn’t offer many integration options, so companies often run separate platforms for artwork and verification. Verify connects directly to document control and artwork tools, keeping quality checks running across the full content lifecycle, not just at isolated points.
Audit-ready reporting: InformaIT produces separate reports for each inspection, which teams must combine manually before submission. That can lead to gaps. Verify keeps everything in one report. It gives teams complete audit trails and submission-ready files without extra formatting. Many teams that move to Verify say they see faster onboarding, better support, and quicker results across departments.
Enterprise support and onboarding: How fast a platform gets up and running matters. Some North American teams have reported slow support from InformaIT, especially during team changes. Verify runs in the cloud and needs very little IT setup. Teams can get started quickly, with help from validation packages designed for fast qualification.
Pricing and implementation
Quality control investments go far beyond license fees. Smart teams look at the complete picture: how quickly you'll start catching errors, what ongoing support you'll receive, and whether the platform scales as your needs evolve.
InformaIT's implementation approach typically involves separate processes for different verification needs. Organizations often need to set up additional components for visual inspection, barcode verification, and Braille validation beyond the core text verification. This modular structure can extend implementation timelines as each component requires its own configuration and validation. The platform's interface with multiple pop-up windows and separate workflows can also extend training times, particularly for teams needing comprehensive packaging verification across different elements.
Verify is ready to use fast, with cloud deployment that doesn’t depend on heavy IT setup. Teams typically begin working in the Verify system within days, not weeks. The platform is easy to learn, and training is built around real use cases. Support teams understand the pace and pressure of regulated environments. That kind of onboarding helps teams get started quickly and use the platform fully across departments.
Teams that implement Verify consistently report not just error reduction, but renewed confidence in their quality processes and the ability to meet increasingly aggressive timelines while maintaining perfect accuracy.
The real ROI emerges when considering error prevention in regulated industries. Packaging mistakes caught during development cost hundreds to fix, while post-production errors trigger expenses in the millions through recalls and compliance penalties. Verify's unified inspection approach catches issues earlier when corrections remain straightforward and inexpensive. Verify’s pricing is scale-based by user count-, not inspection type, so teams avoid budget surprises as needs grow. With these considerations in mind, determining which platform best fits your organization comes down to matching capabilities with your specific verification requirements.
Final decision guide
Quality control decisions shouldn't be complicated. It comes down to what your team actually needs to accomplish and where you're headed.
InformaIT works for teams with narrowly focused text verification needs. If you primarily review regulatory documents, have minimal requirements for visual inspection or barcode validation, and already have established text verification workflows, InformaIT can handle these basic scenarios. Teams who rarely venture beyond straightforward document comparisons might find the platform adequate for their current situation.
Verify excels when you need to eliminate quality bottlenecks across complex packaging and documentation. For teams verifying everything from text and graphics to barcodes and Braille, its unified platform transforms what would otherwise require multiple disconnected systems into a single efficient workflow. Organizations managing multiple markets or languages gain immediate advantages from Verify's comprehensive multilingual capabilities, particularly when scaling quality verification across regions.
For teams ready to accelerate quality processes, Verify's cloud deployment and intuitive interface get your organization up and running in days rather than weeks. Verify connects directly to your artwork and content systems so quality checks happen across the full content lifecycle. This unified approach doesn't just improve accuracy, it accelerates product launches, reduces compliance risks, and turns quality control into a strategic advantage for your regulated content.




