Quality professionals evaluating text verification platforms often focus on InformaIT and Schlafender Hase's TVT. Both have significant market presence, yet many teams discover critical gaps after implementation. The real question isn't which is better, but whether either one actually solves the problems most pharma teams are dealing with.
Regulatory teams face mounting pressure to accelerate review cycles while maintaining perfect accuracy. InformaIT's document comparison tools and Schlafender Hase's TVT platform represent two established approaches to text verification. Both promise to catch errors before they become compliance nightmares, but their approaches differ significantly.
Understanding these differences matters, especially when packaging errors can trigger recalls, regulatory scrutiny, or millions in lost revenue.
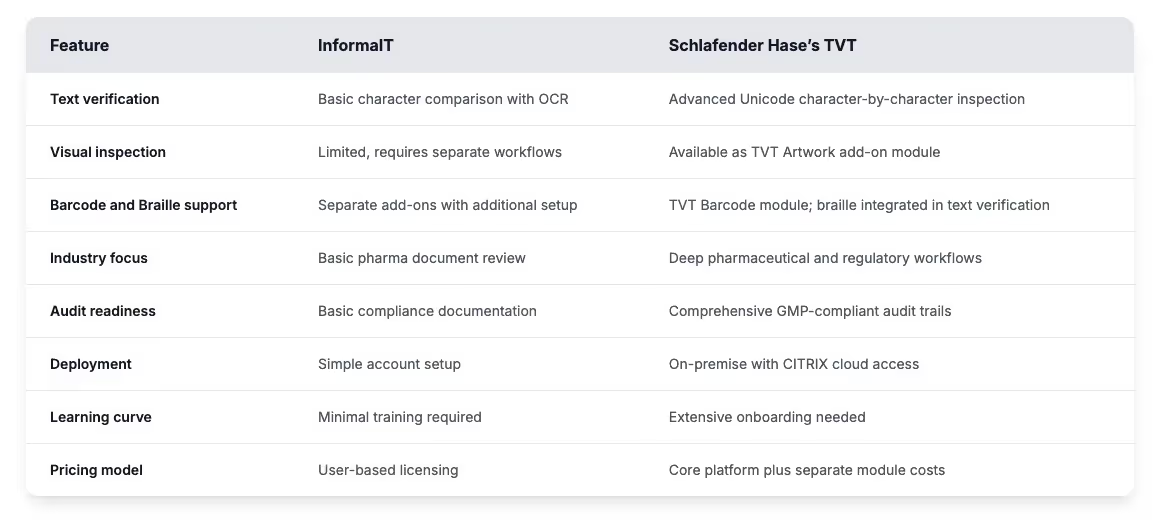
Quick comparison chart
Here’s how InformaIT and Schlafender Hase compare across key inspection and compliance features.
Overview: InformaIT
InformaIT calls itself pharma proofreading software for regulatory teams that need straightforward document comparison. It handles text verification through OCR technology, making it work for teams with basic quality control requirements.
The tool works well for comparing submission documents, package inserts, or patient information leaflets. InformaIT is built for simplicity, making it a fit for smaller regulatory teams with limited technical resources who mostly work with text-based documents. However, teams responsible for packaging and labeling often find it lacks the integrated visual QA needed for full artwork and layout review, making it difficult to verify end-to-end assets like cartons, IFUs, and multilingual labels.
InformaIT’s strength is its approachable interface and minimal technical requirements. Teams can get it up and running without heavy IT support or drawn-out validation processes. But that simplicity also means you’re working with a limited feature set, especially if your QA needs grow over time. The platform struggles with comprehensive packaging inspection that modern pharmaceutical and medical device teams require, often needing separate tools for visual elements, and complex formatting.
Overview: Schlafender Hase (TVT)
Schlafender Hase's TVT is a regulated QA platform in pharmaceutical markets. The platform built its reputation on reliable text verification capabilities that pharmaceutical regulatory teams trust for critical submission workflows.
This reputation comes from TVT's detailed text comparison engine, which performs character-by-character Unicode analysis to detect even subtle changes in regulatory documentation. This precision makes it valuable for teams managing patient information leaflets, instructions for use, and submission materials where accuracy can't be compromised.
Beyond its core text capabilities, the platform offers additional features you can add on. TVT Artwork provides graphics comparison, while TVT Barcode handles barcode grading and verification. This modular approach lets teams scale functionality as needs evolve, though each addition requires separate procurement and validation.
This makes TVT a good fit for pharmaceutical teams that have already validated the platform in their quality systems. Its comprehensive audit trails and documentation capabilities satisfy FDA/EMA compliance tools requirements, especially for teams working exclusively with regulatory documentation. However, the platform’s interface feels outdated, which makes it harder for new users to get up to speed.
Still, teams that work across both regulatory content and packaging files may find its capabilities limited. This is particularly evident when visual components like symbols, barcodes, or layout formatting are part of the QA process.
InformaIT: Pros and cons
A lot of regulatory departments with limited IT resources go with InformaIT because it’s easy to get running. There’s barely any setup, and with minimal training, people can usually figure it out fast, even if they don’t use it daily.
The platform handles basic document comparison effectively, particularly for teams focused on text-heavy regulatory materials. Its OCR capabilities work well for standard pharmaceutical documentation, and the concurrent licensing model can be cost-effective for smaller teams. However, some teams report challenges with onboarding and support. The multi-window user interface can make it difficult to train new hires or scale usage across departments, especially when team turnover occurs.
The problems start when teams need more than basic text comparison. InformaIT can't handle visual inspection, so you're stuck using separate tools for graphics verification. That creates gaps in your packaging and labeling accuracy, slows everything down, and creates opportunities for mistakes when moving files between systems.
The platform's audit trail capabilities fall short of what many regulated QA platforms require. Teams operating under strict GMP compliance often find InformaIT’s documentation insufficient to satisfy audit requirements. While InformaIT integrates with platforms like Veeva and Esko for standard use cases, connecting verification workflows across broader document management systems can be challenging. Support challenges also affect some teams, particularly in North America, and teams often outgrow the platform's basic feature set as verification requirements grow.
Schlafender Hase: Pros and cons
TVT excels at reliable text verification and has deep pharmaceutical industry experience. The platform delivers the precision required for regulatory submission workflows, with character-by-character comparison that catches subtle changes human reviewers might miss. Teams working in validated environments appreciate TVT's established presence and proven compliance track record.
The platform's comprehensive audit trails and documentation capabilities satisfy global regulatory standards, including FDA, EMA, and MHRA requirements. TVT offers additional features as add-ons, starting with text verification, but each added feature requires separate purchase and validation.
Teams hit roadblocks with TVT's clunky design and steep learning curve. The platform requires extensive training for users to become proficient, which can slow implementation and delay time-to-value. Teams often need structured training programs and dedicated resources, making teams dependent on specialized staff.
The modular pricing structure becomes expensive as verification needs expand. Adding features to TVT means buying extra modules, each requiring its own procurement, setup, and validation. That adds both time and cost. Some teams don’t realize this until they’re already deep into a rollout, which can lead to budget issues or missed deadlines. And because TVT relies on an on-premise deployment model with CITRIX access, it’s tough to scale across remote teams.
If neither platform fully supports your quality goals, it might be time to look beyond the usual options. That’s where GlobalVision’s Verify comes in.
There’s another way: GlobalVision
InformaIT and TVT take the traditional approach to pharmaceutical quality control, but GlobalVision's Verify platform fixes the problems both create. With Verify, you don't have to choose between text verification vs visual QA - it handles all inspection types in one platform, running them simultaneously so you can perform all checks at once. You get accuracy, speed, and smart filtering that cuts out false positives.
Verify also gets regular product updates and continues to add AI features to stay current with industry needs.This setup gets rid of the procurement headaches and extra validation work that comes with modular solutions. Teams get text verification, visual inspection, barcode grading, and braille translation as standard features, not expensive add-ons. Verify also handles multi-language documents in one shot, which is critical for global pharma regulatory teams. The platform handles everything from regulatory submission documents to complex packaging artwork within one audit-ready content workflows system.
Verify's cloud setup works across teams, regions, and product lines. You can get it running in days instead of weeks, with validation packages that speed up qualification. Teams see real ROI from their first verification projects, not months down the road. Biogen saved $1.2 million annually after implementing GlobalVision’s tools, significantly reducing their revision cycles and delivering artwork files exactly as intended. Training happens fast with GlobalVision Learn, a built-in learning system that helps teams get up to speed and stay consistent.
The platform connects verification directly to document management systems and artwork approval workflows. This means quality assurance runs throughout your content process instead of just hitting isolated checkpoints that can miss things. Pharmaceutical, medical device, and CPG companies worldwide use Verify to meet global regulatory standards. But looking at how these three approaches compare on key evaluation criteria helps you figure out which fits your specific needs.
Comparison: What to look for in any proofreading tool
Both InformaIT and TVT handle text verification well, but they take different approaches to comprehensive packaging inspection. Good pharma proofreading software needs to handle more than just regulatory text - visual elements, symbols, and formatting all affect compliance.
FDA/EMA compliance tools need comprehensive audit trails that track every user action and document change. Teams need platforms that generate submission-ready reports without manually pulling together data from multiple systems. Being able to export documentation in regulatory-standard formats saves hours during inspections and helps speed up approvals.
Integration with existing workflows makes the difference between platforms that help productivity and those that slow things down. Quality control works better when it connects to document management, artwork approval, and content lifecycle systems rather than sitting by itself. Teams working across departments need platforms that tie verification into their broader regulated workflows.
Support and training make a big difference for implementation success and whether teams actually use the platform long-term. Platforms needing extensive technical expertise slow deployment and make teams dependent on specialized staff. Teams need solutions with intuitive interfaces, good documentation, and responsive support that understands regulatory timelines.
Long-term scalability means your quality platform can grow with changing compliance requirements and expanding product portfolios. If you’re managing multiple markets, languages, or product lines, you need a platform that can handle that complexity without piling on administrative work or causing validation headaches.
With that in mind, let’s break down which type of team each platform really fits best.
Final decision guide
Think about your verification needs, how your team is set up, and where you’re headed. Those three things should drive your decision. Each option serves different organizational needs and compliance scenarios.
For small teams with basic needs, InformaIT makes sense if you manage straightforward document comparison with minimal visual inspection requirements. The platform works well for organizations focused primarily on text-heavy submission materials. Teams comfortable with basic compliance documentation and simple deployment may find InformaIT adequate for their current scope.
Teams with established pharma processes often gravitate toward Schlafender Hase’s TVT, especially those wanting to stick with deep pharmaceutical industry focus. The platform suits teams with established validation processes who value proven regulatory compliance and can invest in comprehensive training. Organizations comfortable with modular expansion and complex implementation might like TVT's specialized approach.
Most teams today need something more complete. GlobalVision’s Verify works well when you need packaging and labeling accuracy across every element - text, graphics, barcodes, and braille. The platform handles complex verification requirements, tight timelines, and cross-departmental quality initiatives. Organizations looking for modern deployment options, intuitive interfaces, and global regulatory standards compliance find Verify changes quality control from bottleneck to advantage.
Some tools handle just one part of quality control. But if you're managing life sciences quality across multiple areas, you need a platform that covers everything without slowing you down. As regulatory requirements keep changing and packaging complexity increases, the platform you pick today should support tomorrow's compliance demands.




