Digital Transformation for Pharmaceutical Packaging Quality

Introduction
The globalization of the entire pharmaceutical industry has led to differing requirements across many countries, furthering the need for compliance standards as well as high-quality packaging.
Now more than ever, pharmaceutical and life science companies must comply with an extensive array of regulatory requirements and standards that are created by governing bodies like the FDA and EMA. These standards outline specific guidelines and obligations that pharmaceutical companies must adhere to if they wish to operate on a global scale.
As the demand for safe medical drugs increases around the world, so does the responsibility of regulatory affairs personnel who must ensure that the guidelines received from governing bodies are successfully interpreted, applied, and communicated within the company. This is essential in ensuring that their products meet the required standards for sale in diverse parts of the world. Localization, or the process of tailoring a product to meet both legal and cultural requirements of a specific market, has become an integral part of the role of regulatory affairs and has expanded far beyond translations.
This comprehensive guide from GlobalVision will analyze and discuss how the increasing adoption of technological solutions is the key to managing localization, regulatory compliance and packaging quality while ensuring that pharmaceutical companies have the tools they need to operate at scale.
The many components of localization
All aspects of pharmaceutical production including regulatory affairs, packaging, and post-marketing compliance must adhere to the highest standards of quality control established by agencies like the FDA and EMA. As globalization continues to expand market sizes, pharmaceutical companies must coordinate localization for every product they release. They must ensure that their products are communicated and made available in multiple languages while adhering to the directives and compliance requirements of every region in which they operate.
Aside from translations, localization includes adjusting color preferences, units of measurement, date formats, artwork, and other elements to reflect various markets. In the pharmaceutical industry, more information is produced at each stage of the product’s life cycle than any other market segment. Legal documentation, leaflets, cartons, inserts, and all other content must be developed to fit the unique needs of a particular geographical area, posing challenges for regulatory teams when it comes to ensuring content accuracy across each stage of the workflow.
Furthermore, the speed at which new drugs must be released makes it difficult to ensure that no errors have been introduced when adapting content to local markets. This adds tremendous complexity to a process where the margin of error is already small as failure to localize correctly can lead to potential legal issues and decreased revenue from overseas markets.
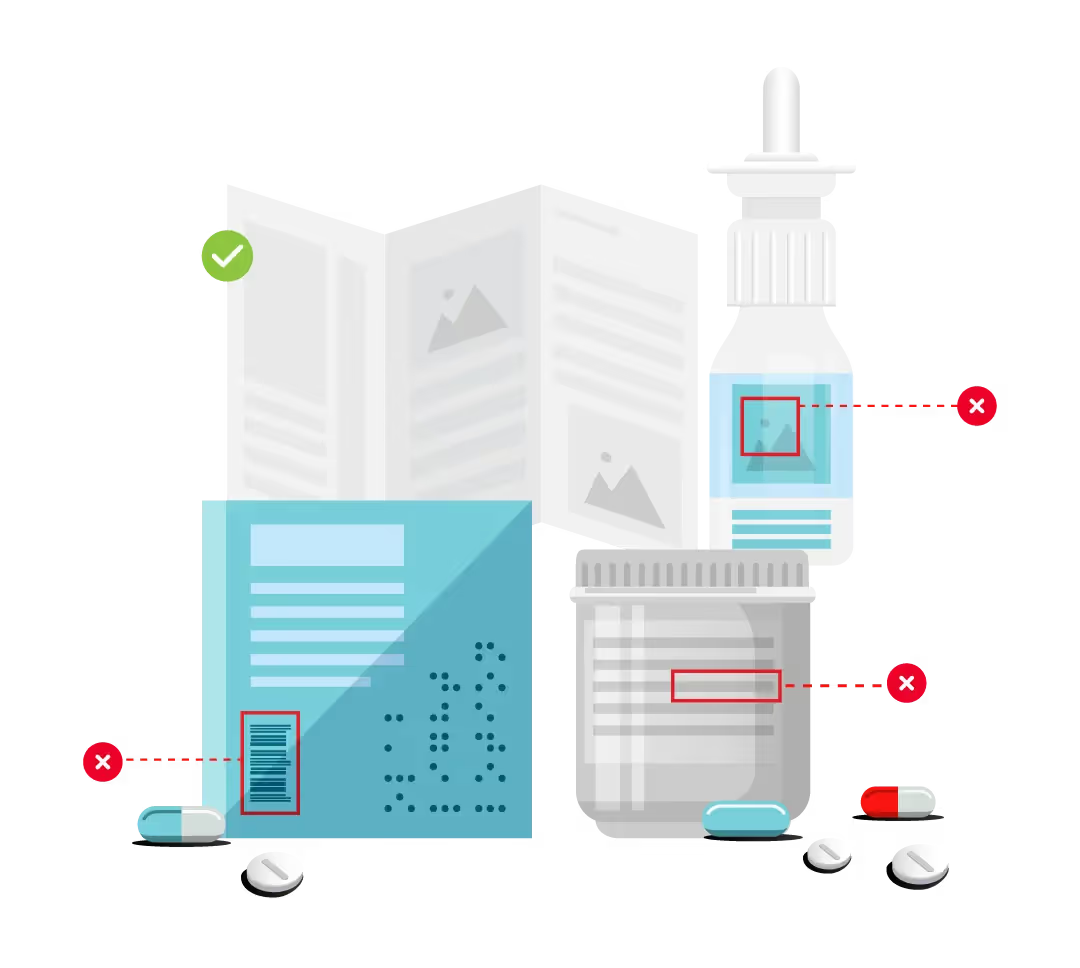
Packaging errors have become a leading cause of recalls in the global pharmaceutical market as product packaging continues to be adapted to meet localization requirements. From spelling errors that appear when translating foreign languages to artwork defects, the risk of letting a pack- aging error slip by is high. As market size increases and pharmaceutical companies become more dynamic, regu- latory workloads become even more complex and difficult to manage. Skilled workers are often in high demand and short supply, creating room for error in areas such as compliance, artwork management, and labeling. To help deal with this increasing trend, more and more companies are introducing digi- tal solutions across several aspects of the pharmaceutical workflow.
Using technology to operate at scale
One of the biggest challenges faced by quality control and regulatory professionals is the amount of data they must manage on a daily basis. Regulatory affairs play a critical role by acting as a link between health agencies and various departments within the company to ensure that all the data accumulated is properly transcribed to create and market the drug. From obtaining approval for a new product to ensuring that the correct FDA guidelines are applied to labeling into terms of content and formatting, the regulatory affairs department has a hand in many different aspects of production. Additionally, reviews must be done at every stage of the file creation workflow to ensure that the information remains accurate and compliant with what has been approved.

A key way to effectively manage the increasing workloads as a result of globalization is to harness technology solutions that allow regulatory affairs in the pharmaceutical industry to operate at scale. When it comes to proofreading, manual processes are no longer feasible given the size and speed in which products must be delivered. A digital quality control system can be used to verify content accuracy on digital files and printed packaging materials, eliminating the need for manual proofreading and the inefficiencies that come with it.
Digital proofreading for the pharmaceutical workflow
Automated proofreading can be implemented at every stage of the pharmaceutical workflow to ensure that no errors have been created or introduced. To begin, product and drug requirements are submitted to regulatory bodies who then compile the information into briefing documents for the appropriate regulatory agency to review. The implementation of a digital quality control system at this stage would ensure that all copy documents are error-free and accurate for internal revisions and regulatory submissions by automatically inspecting text-heavy documents character-by-character. At the artwork creation stage, artwork files can be automatically inspected to make sure that no errors were introduced during the file creation process. These errors oftentimes include compliance mistakes like logo errors, incorrect information, and barcode defects. Digital inspections can be continued as the files are edited to ensure that no unintended errors have been added.
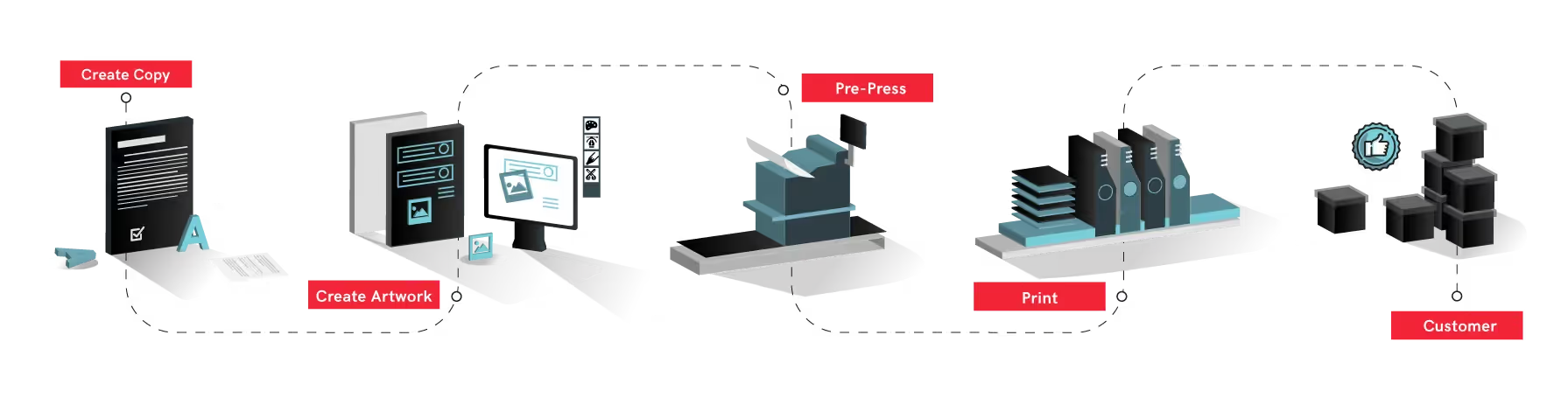
It is imperative that content remains accurate at every stage of the process, across all departments. A digital proofreading solution also helps to keep track of version changes between departments, reducing long approval cycles and time-to-market. At the final stage, digital quality control software can automatically compare approved files to supplier proofs before going to print, ensuring that products make it to market with packaging that is entirely error-free. Digital systems further improve the overall efficiency of the pharmaceutical packaging workflow as they cut down on revision cycles and catch errors earlier in the process, no matter how small the errors are.
“To prevent recalls and ensure good labeling quality that stays in compliance… you can’t handle it on your own. You need a tool like GlobalVision not only to be faster but safer too.”
Caroline Sell, Regulatory Affairs Manager Merz Pharmaceuticals
Another challenge for regulatory professionals in the pharmaceutical industry is the management of content in foreign languages. As medical products continue to make their way into foreign markets, packaging, text- heavy inserts, and IFUs must be translated into various languages. Translations are often outsourced to external companies who can translate upwards of 2000 words per day, leaving significant room for error. Technology solutions can help effectively manage this by improving document management, version control, and quality assurance through automated proofreading that supports many foreign languages.
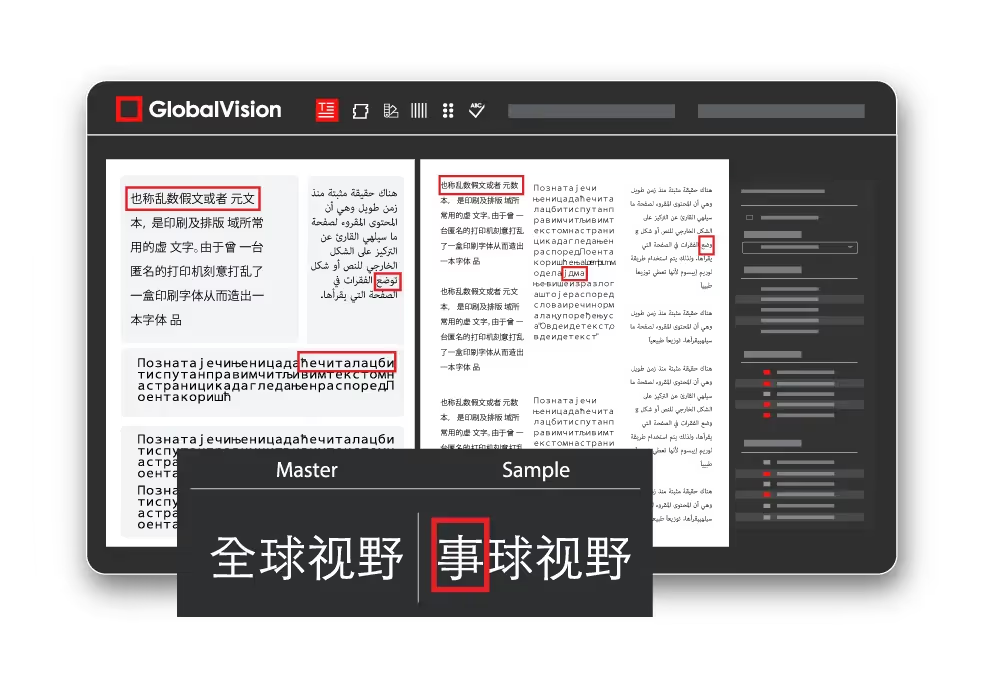
In the pharmaceutical industry, compliance and data integrity guidelines are essential. Raw data should be retained in the event of investigations. Digital quality control tools utilize audit trails to track all activities, ensuring compliance with bodies like ISO certifications, 21 CFR Part 11, and Annex 11. Audit trails are important as they are the key to maintaining data integrity by tracking all activity, including inspections and reports. Comprehensive inspection reports help protect data integrity by providing full traceability on who inspected a product and what the results of the inspection were. These reports keep track of and review all found differences between files, ensuring that the right changes are made.
Conclusion
There is no doubt that digital solutions act as insurance against potentially catastrophic errors like missing decimal points in dosage figures, the value they provide to pharmaceutical companies extends far beyond eliminating manual proofreading. As the nature of regulatory standards continues to evolve, so does the need to effectively implement technological solutions like automated quality control. These technological innovations not only improve processes between teams, but they also increase cross-functional collaboration and give pharmaceutical companies the tools they need to be able to operate on a global scale.


