The Rise of Automation Technology in Regulatory Affairs
Executive Summary
Emerging technologies allow pharmaceutical companies to accelerate product development and commercialization faster than ever before. This report discusses how technology, specifically automation and digital technologies, impact regulatory affairs in the pharmaceutical industry.
The findings concluded that automation and digital technologies help regulatory professionals significantly reduce proofreading and review time, minimize revisions, and get products to market faster.
Additionally, the evidence shows that these technologies help regulatory professionals be more cost-efficient when scaling manufacturing, ultimately benefiting their bottom line.
Finally, substantiation supports that automation and digital technologies help ensure consumer safety advisories are in place.
Looking toward the future, the research forecasts with reasonable certainty that automation and digital technology will continue to grow across all departments in the pharmaceutical industry while significantly influencing the future global alignment of regulatory affairs.
Navigating The Regulatory Affairs Landscape
Every country has a regulatory agency in place to ensure consumers purchase trusted and reliable health products. The United States Food and Drug Administration (FDA) holds an Office of Regulatory Affairs (ORA) to regulate products related to public health.1 Similarly in Canada, Health Canada modulates products through their Health Products and Food Branch (HPFB). 2 In the European Union, the European Medicines Agency (EMA) spearheads the region’s medicinal product lifecycle. 3 With regulatory agencies in place, fewer medication errors—cases of improper medicine usage—can occur.
Still, statistics show a different picture. An FDA statistic from Harvard Health Publishing reports that the agency experienced over 14,000 drug recalls in the past decade, equating to an approximate average of four drug recalls per day.4

Companies rely on regulatory affairs professionals to ensure products comply with legislative requirements. However, high volumes of pharmaceuticals are continuously being shipped to market with errors including incorrect dosage labeling. To combat this, companies utilize innovative automation and digital technologies to streamline regulatory processes across workflows that otherwise require numerous manual inspections.
This report navigates how regulatory affairs professionals in the pharmaceutical industry utilize these technologies in their effort to remain compliant and protect consumers.
Methodology
Our research consisted of mixed methods, studying subjects that specialize in regulatory affairs.
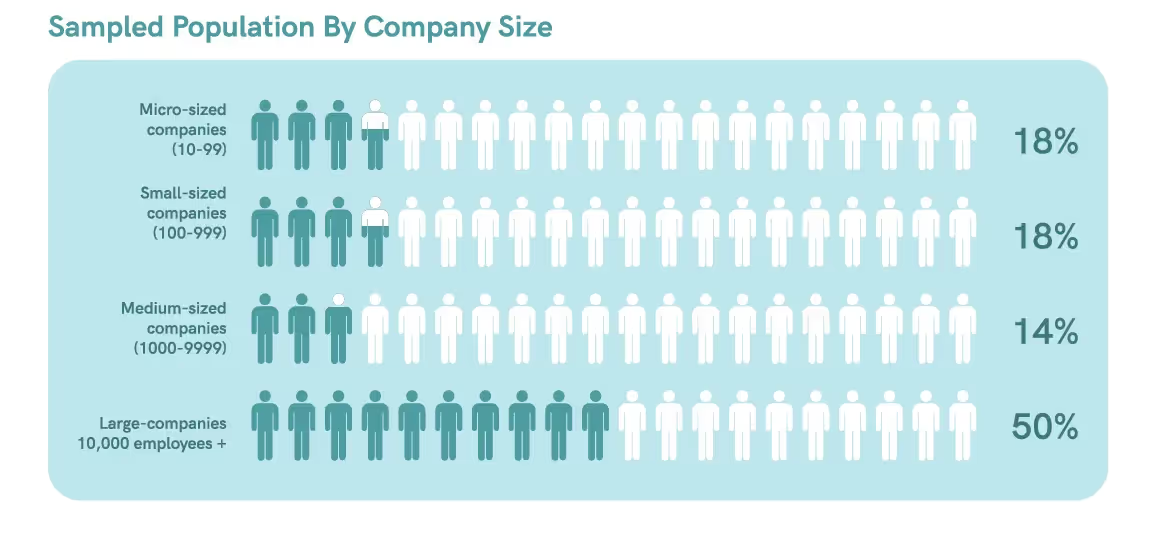
Our survey collected data from 28 diverse regulatory affairs professionals spanning various-sized companies. The survey identified trends regarding the impact of automation and digital technologies on compliance procedures and success metrics.
Demographics

Geographical Scope
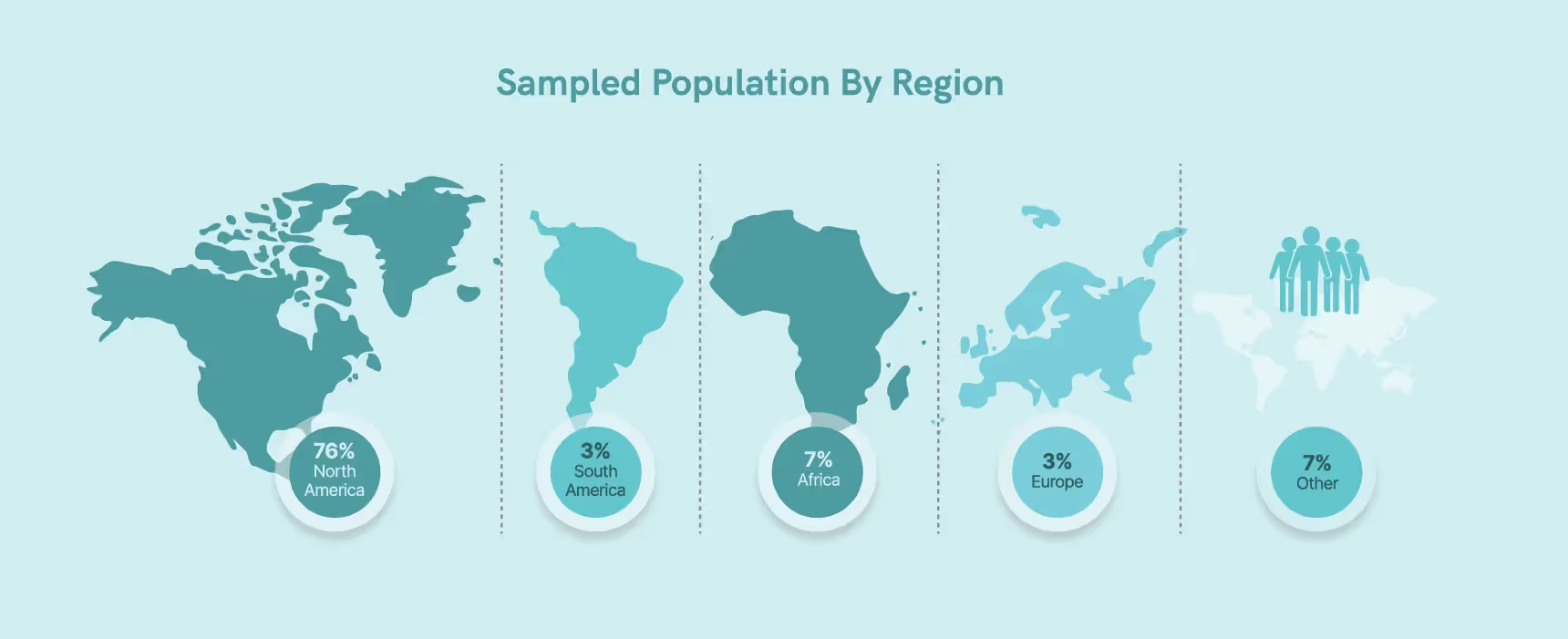
Our focus on the industry is primarily in the Western region, with 76% of our respondents residing in North America (the remaining respondents were as follows: 7% Africa, 3% Europe, 3% South America, 7% Other).
Similarly, North America and Europe comprise approximately 66% of the global pharmaceutical market, according to a RAPS (Regulatory Affairs Professionals Society) Report from 2021.5
Additionally, we conducted external market research from a carefully curated library on a similar demographic which macroeconomically impacts the industry.
Understanding the Pharmaceutical Industry
Before delving further into Regulatory Affairs, a prerequisite is to understand the pharmaceutical industry. Which is responsible for the development, production, and distribution of healthcare drugs, operating within a complex regulatory framework.
Market Insights
Despite its complexities, the global pharmaceutical industry continues to grow rapidly, with the most commercially successful pharmaceutical drugs each boasting the ability to generate over $1 billion USD per year.6
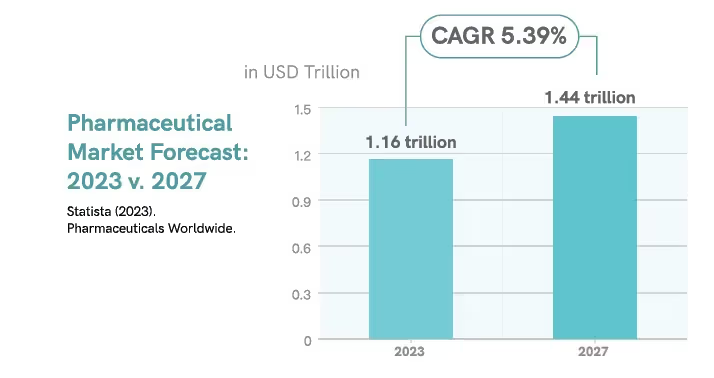
The pharmaceuticals market is projected to reach a market volume of
$1.16 trillion USD in 2023, growing at a compound annual growth rate (CAGR) of around 5.39% to reach $1.44 trillion USD by 2027.
Although these forecasts seem promising, current pharmaceutical trends shed some concern on the industry’s variable costs and savings opportunities, such as users’ adaptation to technological advancements, labor market challenges, and inflation.8

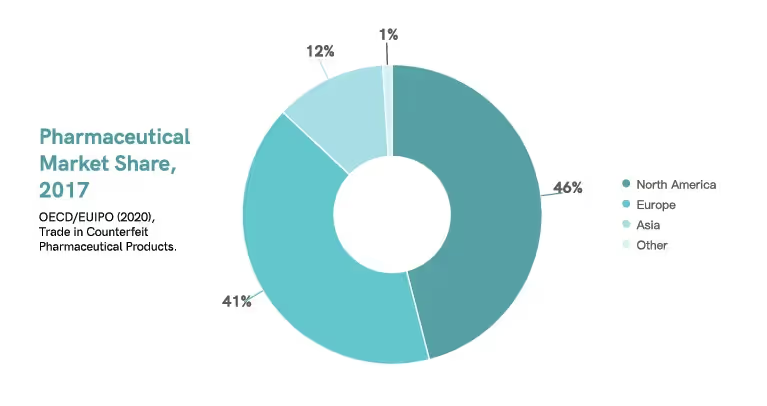
Based on a 2017 study, North America saturated about 46% of the global pharmaceuticals market, followed by Europe with about 41% and Asia with about 12%.9 Currently, the United States and Europe dominate the global pharmaceutical market.5
Business Categories
The industry is characterized by various companies specializing in healthcare, life sciences, and biotech practices. These include:
- Research-based Pharmaceutical Companies
- Generic Pharmaceutical Companies
- Contract Development and Manufacturing Organizations (CDMOs)
- Biopharmaceutical Companies
Research-based Pharmaceutical Companies

These companies focus on research and development (R&D) to discover and develop new pharmaceuticals and invest heavily in scientific research and clinical trials.
Generic Pharmaceutical Companies

These companies produce and market generic versions of brand-name drugs after the expiration of patent protection. They aim to provide more affordable alternatives to brand-name drugs.
Contract Development and Manufacturing Organizations (CDMOs)

These companies offer outsourced services for drug development, formulation, manufacturing and packaging for pharmaceutical companies.
Biopharmaceutical Companies

These companies focus on developing drugs derived from biological sources, such as living organisms, cells, proteins, and antibodies.
The industry continues to evolve with emerging hybrid models of the above companies. Therefore, these company types are not mutually exclusive.
Pharma’s Stakeholders Go Beyond the End Consumer
Pharmaceutical companies are responsible for satisfying three main customer groups:10
- Patients: Due to regulatory constraints, pharmaceutical companies have limited opportunities to engage directly with patients.
- Patient-facing Staff: These include general practitioners (GPs),
specialized physicians, pharmacists, and nurses.
- Regulators: These regulatory agencies include FDA and Health Canada.
Subject Spotlight: The Regulatory Affairs Professional
The regulatory affairs department ensures pharmaceutical products’ safety, efficacy, and quality.11 Of over 98,000 regulatory professionals worldwide, 65% specialize in monitoring pharmaceutical products.5
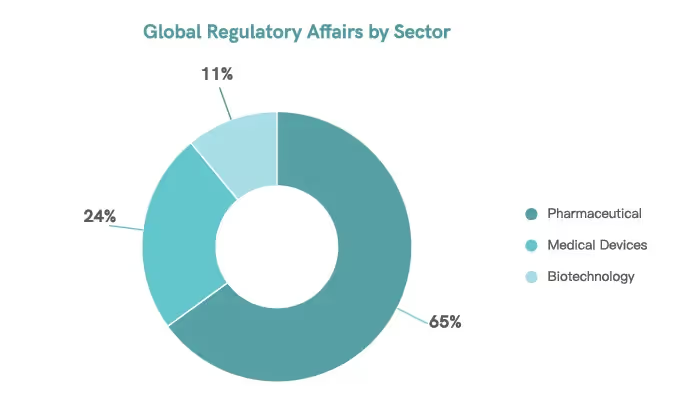
Regulatory professionals play a vital role in navigating the challenges of global regulatory requirements and ensuring that companies comply with standards, becoming the cornerstone of product development and commercialization. Through their work, companies are able to safeguard their products and improve public health.11
Most Regulatory Affairs Departments Are Found in Enterprise-Level Companies
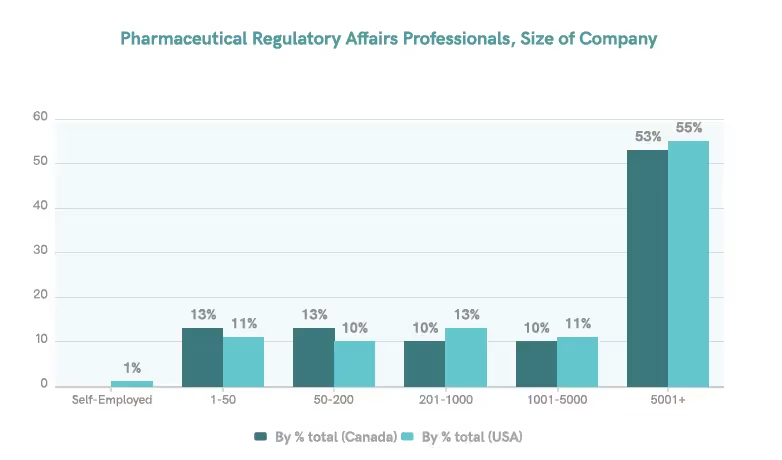
In Canada and the United States, most regulatory professionals are employed by enterprises, with about 53-55% of them working for organizations with over 5,000 employees.5 This indicates that our survey sample is an adequate representation of the target population.
Regulatory Teams Are Small But Mighty With the Help of Automation
Our survey shows that regulatory teams comprise an average of 10 people.
Before COVID-19, the majority of regulatory professionals worked onsite. However, pharmaceutical companies are now adjusting to the “new normal” of a hybrid work model due to numerous work-from- home benefits. Throughout this transition, cloud-based technology played a vital role in team communication and document management, as teams needed an efficient and secure way to keep up ‘business as usual’ in their new remote work reality.12 Still, technology plays a vital role in automating regulatory procedures.
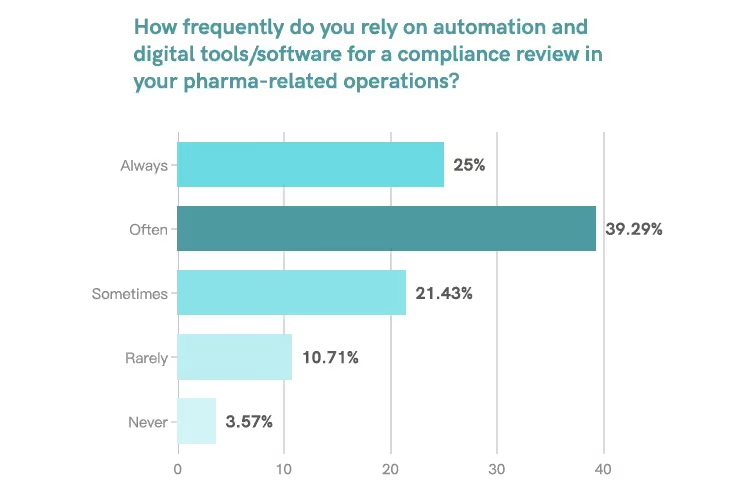
When asked, “On a scale of 1 ‘Never’ to 5 ‘Always’, how frequently do you rely on automation and digital tools/software for a compliance review in your pharma-related operations?” The survey shows that:
- 39.29% of regulatory professionals chose 4 (Often).
- 25.00% chose 5 (Always).
This means that over 64% of respondents frequently used automation and digital tools for their work.
To ensure product safety and mitigate the risk of financial losses caused by errors, regulatory professionals collaborate on various documents. 46.63% of respondents’ answers included labels, followed by 21.43% having product content, 14.29% included leaflets, and 14.29% included packaging content.
Regulatory professionals monitor product labelling and descriptions to ensure they comply with patient safety and regulations.
Technology Expedites Compliance
Regulatory professionals ensure pharmaceutical product safety through compliance measures. Ideally, 100% accuracy in documentation ensures pharmaceutical companies mitigate the risk of additional expenditures due to errors, such as fines and penalties.

This number does not include other highly regulated products produced by the industry such as medical devices, vaccinations, controlled substances, and dietary supplements, which undergo similar rigorous compliance and quality processes, which speaks to the scale of the landscape.
Still, the process of manufacturing these products continues to be complex, time-consuming, and expensive—highlighting regulatory professionals’ greatest fears: noncompliance issues such as recalls, legal disputes, sanctions, fines, and consumer complaints.11
Since 2000, the increase in compliance regulations has complicated companies’ already complex portfolios, though not without reason. The risk of errors brings large costs including:14
- A Corrective and Preventative Action (CAPA) costing up to
$10,000 million USD.
- Warning letters ranging from $2 to $20 million USD depending if production changes are required.
- Constant decrees reaching up to $100 million USD.
To mitigate these costs, regulatory teams routinely review a variety of highly sensitive documents—such as product formulas, Instructions for Use (IFUs), labels, and cartons—and are often performed manually or visually. These review processes are meticulous and time-consuming, with the goal of maintaining compliance and constant monitoring to ensure that only intended changes have been introduced.
Today, technology allows for improved review methods:

Technology Optimizes Regulatory Success Metrics
Quality metrics play a critical role in assessing and improving the effectiveness, efficiency, and compliance of regulatory processes within the industry. They also provide quantitative measures to evaluate
the manufacturing and distribution performance of pharmaceutical products.
Maximizing Time-Efficiency
According to our survey, automation and digital technology have helped regulatory professionals maximize various Key Performance Indicators (KPIs), with 60.71% of respondents saying they have become more
time-efficient with the implementation of technology.
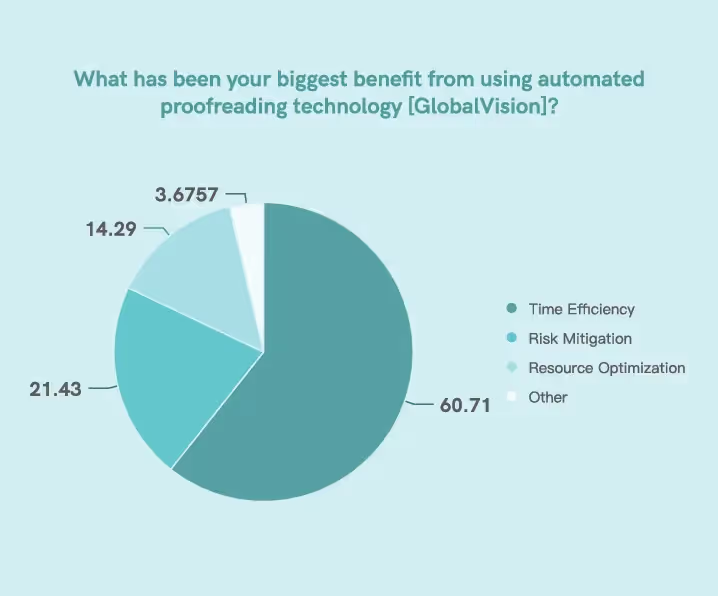
Accelerating Regulatory Submission Timelines
Regulatory Submissions Timelines: Automation is increasing productivity in regulatory submissions.11 The metrics collect the amount of time to prepare and submit regulatory documents, such as Investigational New Drug (IND) applications, New Drug Applications (NDA), and Marketing Authorisation Applications (MAA).
Mitigating The Risk of Non-Compliance
Compliance Monitoring: Automated technologies allow regulatory professionals to capture larger amounts of data and take regulatory actions faster.11 Issues can include deviations, non-compliance errors, and corrective actions for adherence to regulatory requirements and industry standards.
Enhancing Regulatory Framework Effectiveness
Thanks to technology and innovation, most respondents say they were able to focus on more projects and human-centric tasks.

Opportunities For Even More Efficiency With AI
In their reviews, regulatory agencies have increasingly focused on quality systems and process maturity.15
Today, artificial intelligence (AI) and machine learning (ML) are the primary technologies used for compliance procedures.16 AI and ML analyze large sets of data and extract patterns and predictions that can be reiterated in regulatory processes. AI and ML help regulatory professionals identify anomalies and non-compliances issues, and even automate repetitive tasks.
Moreover, AI and ML help automate the following:16
- Risk Assessment: Prescribing compliance-related risks in data following creating a correction plan.
- Crisis Management: Identifying potential security incidents, such as data breaches, and how to counter them.
- Predictive Analytics: Forecasting long-term trends of compliance risks, their impact, and how to prevent and mitigate them.
Future Outlook: Automation Beyond Compliance
Compliance-Boosting Technologies to Continue Expanding
Looking ahead, automation and digital technology will continue to benefit regulatory affairs professionals in the long term. Regulatory professionals are increasingly embracing automation technology and digital solutions to enhance their processes.
By 2030, regulatory technology will significant increase in market demand.17
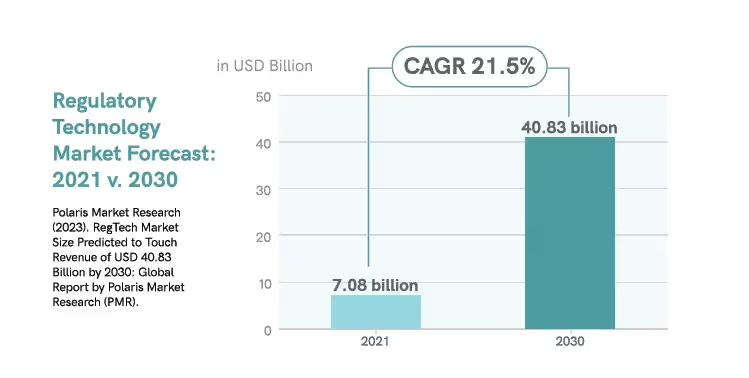
As the regulatory landscape evolves, the industry will continue to explore other applications for automation technology.
Compliance is predicted to extend beyond audits and revisions and be implemented into business activities.16 Compliance automation will be coupled with other departments to modify policies and make data-centric decisions. This will drastically minimize the risk of non- compliance at an enterprise level.
Harmonization of Global Regulations And Stricter Guidelines
As pharmaceutical research continues to expand, regulations are predicted to become even more strict. However, in the next 10 years, regulatory professionals predict there will be more global alignment in regulations—technology can spark opportunities across the regulatory end-to-end value chain to improve the speed, accuracy, and quality of regulatory activities.11
This prediction aligns with one European study of 217 respondents showing that 53% of pharmaceutical companies were negatively affected by BREXIT in 2020.18
In the future, technology is expected to bridge communication gaps between global regulatory agencies and simplify the approval process.


