Solving The Content Efficiency Problem in the Pharma Industry
The Global Importance of the Enterprise Pharmaceutical Industry
In its essence, the enterprise pharmaceutical industry deals with the discovery, development, and manufacturing of drugs and medications for both private and public entities and organizations.
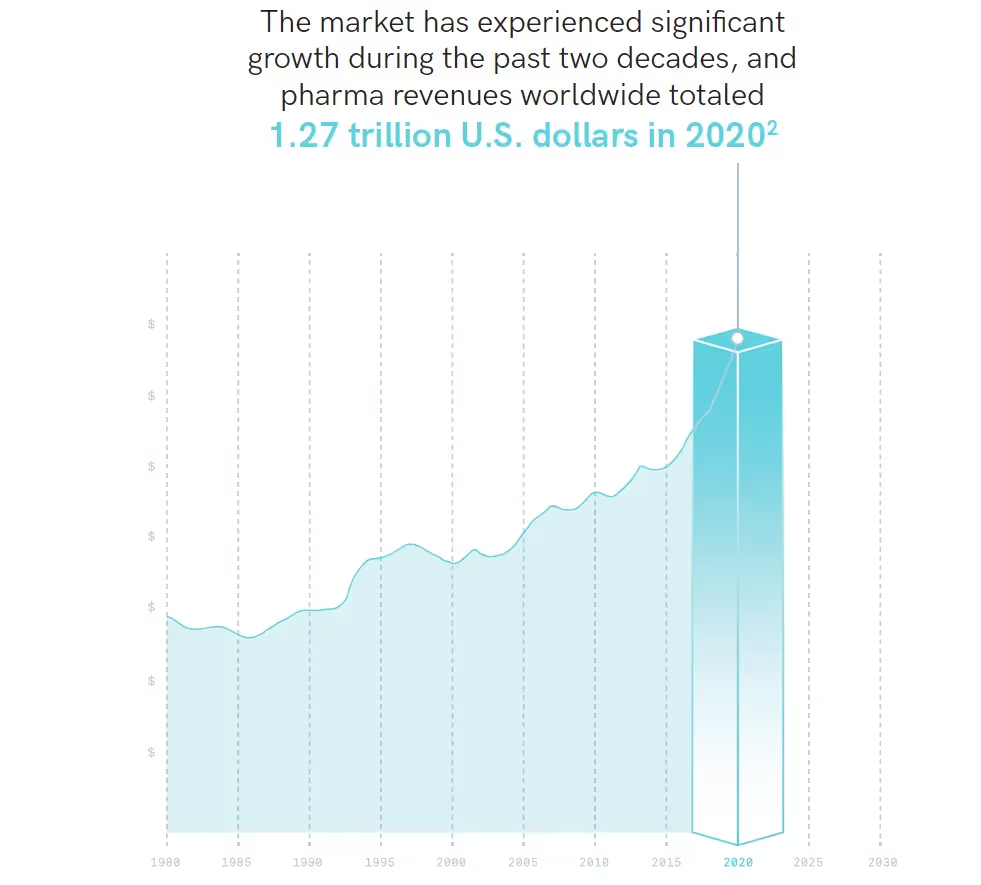
From everyday over-the-counter drugs to highly complex medications for specific ailments and treatments, the research, creation, and subsequent distribution of these drugs has become one of the largest global industries, grossing over 1.27 trillion U.S. dollars in 2020 alone, with steady annual growth.1
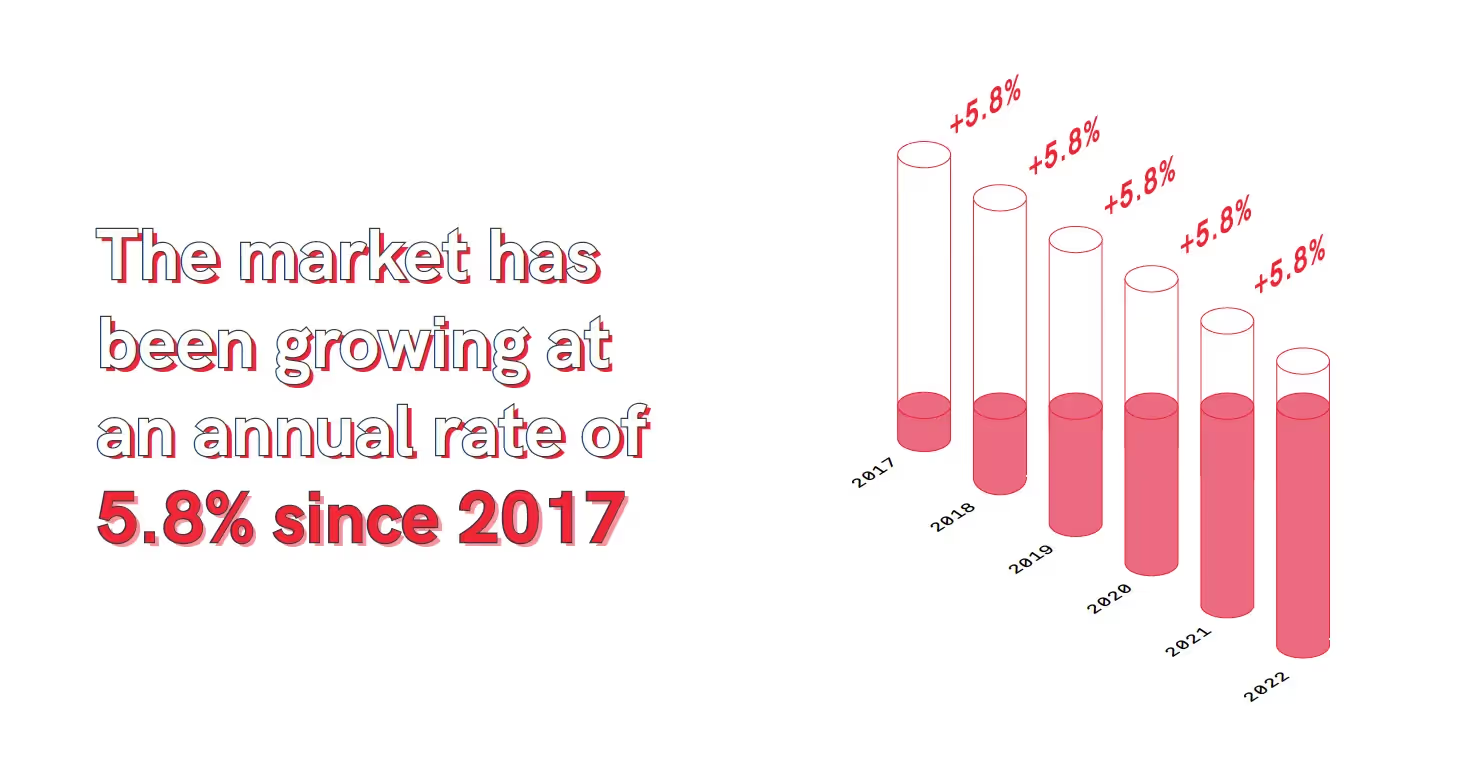
The industry is also solely responsible for major global economic gains and turnover as it creates various new market opportunities and is also expected to grow exponentially in the coming decades. In fact, the market has been growing at an annual rate of 5.8% since 20173 and is arguably one of the most important industries on the global market, encouraging the economy through jobs, products, and profits.
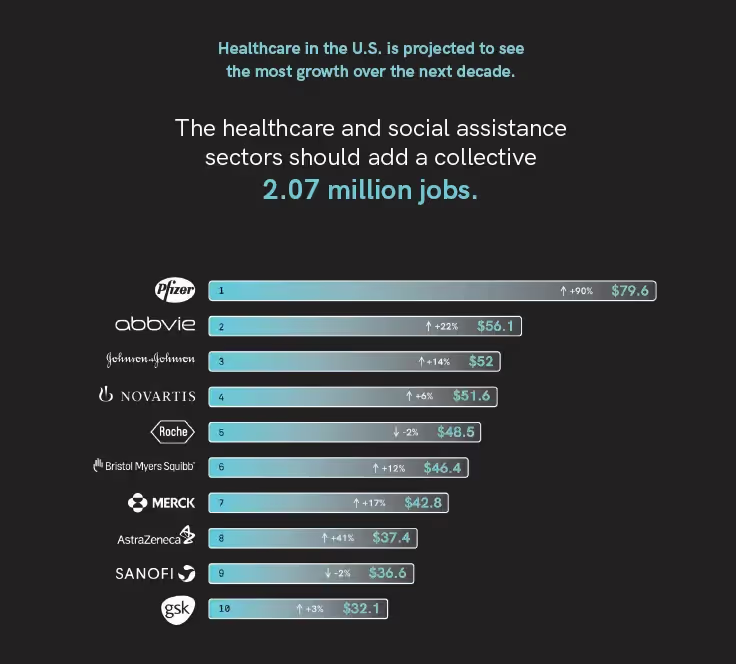
In the U.S. alone, healthcare is projected to see the most growth over the next decade and social assistance sectors should add a collective 2.07 million jobs to the market.4
The pharmaceutical industry is one of the most important industries on the global market and will continue to be so in the future. Yet, due to the nature of its products and services, it is also the most highly regulated industries as laws regarding the development, creation, and distribution of pharmaceuticals are highly monitored and standardized. The documentation and content surrounding pharmaceuticals need to be extremely accurate, error-free, and of the utmost quality.
The accuracy of its instructions, packaging, and labeling must be extremely accurate to avoid life-threatening side effects and colossal financial downfalls. Any errors or recalls can lead to worst-case scenarios such as potential life loss but can also lead to negative brand image and reputation which is particularly harmful to pharmaceutical companies that rely on customer loyalty as a major selling point of their highly specialized products.
With so much on the line, pharmaceutical companies cannot risk errors slipping through that could have detrimental consequences to the organization and its consumers as a whole.
Drug Regulations within the Industry
By definition, pharmaceutical and medicine regulations are the combinations of legal, administrative, and technical measures that governments take to ensure the safety, efficacy, and quality of medicines, as well as the relevance and accuracy of product information.5
Regulations vary from country to country as each individual government has reserved the right to regulate the industry as they see fit. Regardless of the agency, organization, or administration, there is a constant among them all. Their main task is to ensure the utmost quality, safety, and efficacy of the drugs that make it to market, along with the accuracy of their product information.
For example, in the U.S., the lack of adequate drug control systems or methods to investigate the safety of new chemical compounds became a great risk as prefabricated drug products were broadly and freely distributed. Because of this, the US Pure Food and Drugs Act against misbranding was passed in the early 1900s. The Act required improved declaration of contents, prohibited false or misleading statements, and required content and purity to comply with labelled information.6
Due to the sensitive nature of the products, laws and practices within the industry need to take extremely low-risk approaches to the production and distribution of their products. This subsequently makes the pharmaceutical industry one of the most highly regulated industries on the global market.
Because of this, practices and work flows need to be extremely efficient with the smallest possibility for error. The seemingly simple mistake of a typo, an added or removed letter, number, or hyphen can have catastrophic consequences not just for the company but for consumers as well.
Ultimately, drug regulations ensure the safety of the products itself, but who is ensuring that the content of the written copy and artwork is of the highest standard?
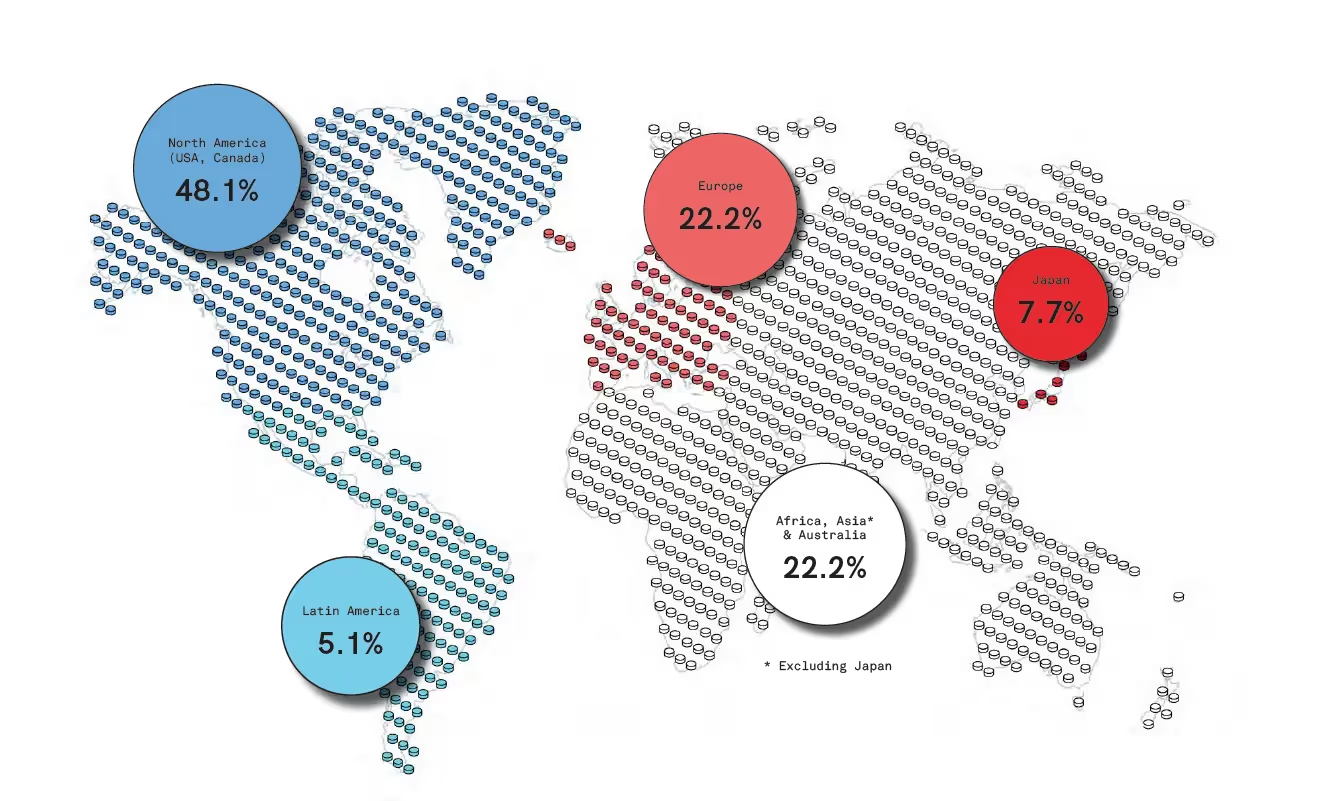
Breakdown of the global pharmaceutical market
The Pharmaceutical Industry has an Efficiency Problem
In such a highly regulated industry, it would be assumed that efficient and streamlined workflows are already in place to ensure that the content behind every product is accurate and error-free.
Unfortunately, that’s not always the case.
Errors in pharmaceutical packaging, labeling, and product descriptions are still regular occurrences that cause detrimental consequences from consumer’s well being to the integrity of the products and company.
In fact, it’s estimated that 50 percent of all pharmaceutical recalls are related to errors in labeling or packing artwork.8

It is also important to note that recalls have a large financial impact on the healthcare system and they are a lot more frequent than one would imagine. Clinically important drug recalls occur approximately once per month in the United States. For perspective on just how large these financial impacts can be, Johnson and Johnson lost approximately $600 million in sales after closing a distribution site due to a recall.9
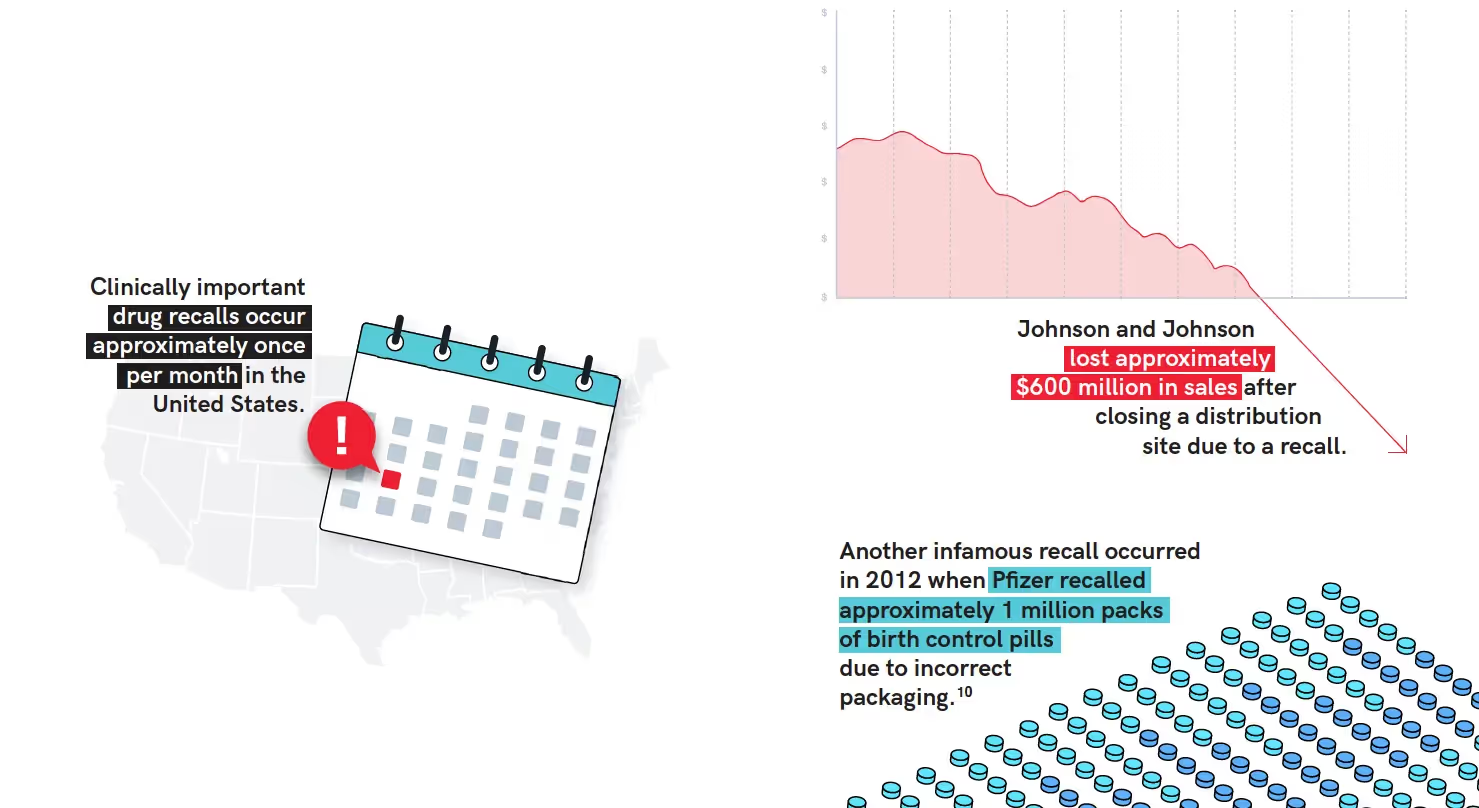
Overall, in the pharmaceutical industry, the top recall causes are incorrect labeling, defective products, and incorrect potency.11 Common mistakes in packaging and labeling are typically necessary information missing from artwork, content errors, and technical errors such as flawed barcodes.
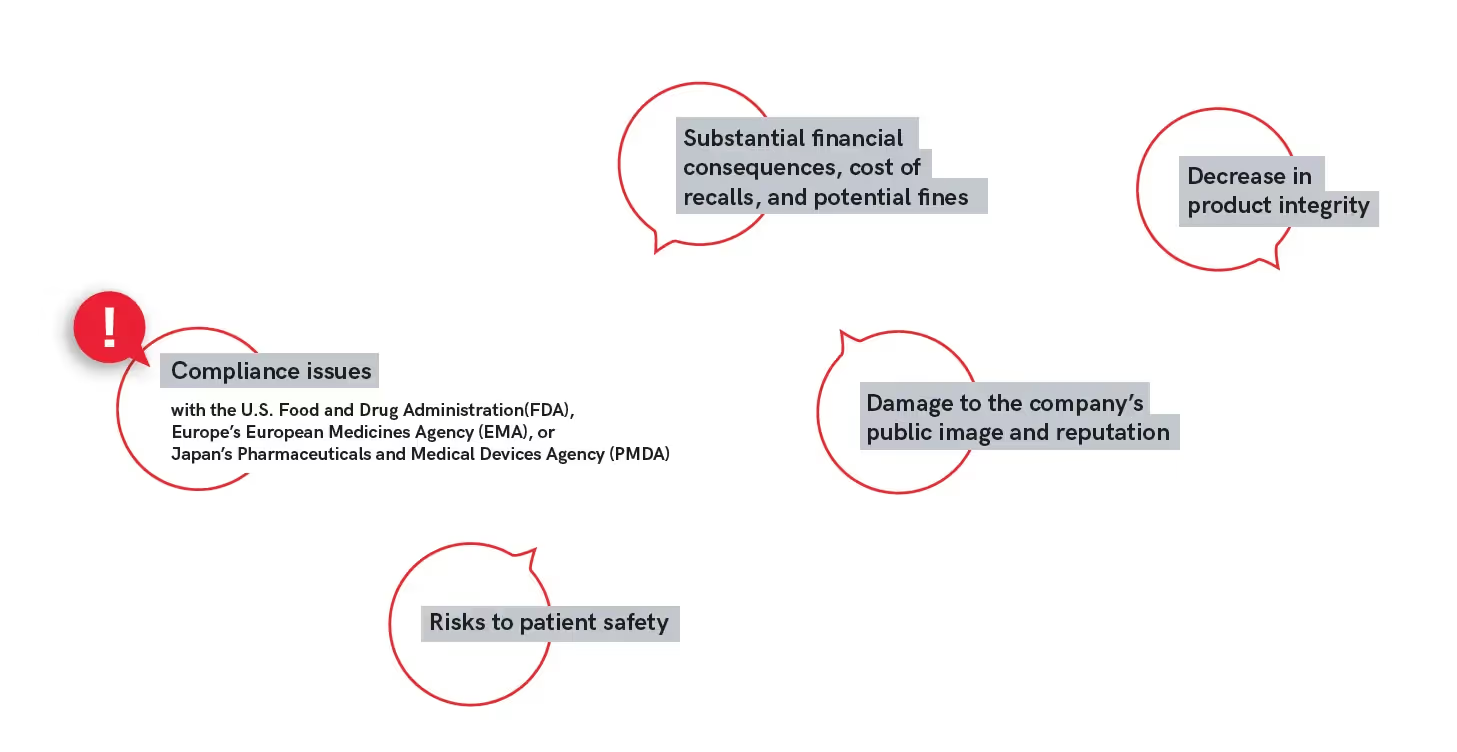
These errors in documentation, print, and packaging can result in a multitude of problems including:
The Proofreading Lifecycle
To develop, produce, and distribute pharmaceuticals, a complex product lifecycle exists that includes an intricate reviewing and proofreading process; a process that is currently flawed with immense potential for improvement.
Global corporations are currently faced with challenges at each stage of product proofreading:
- Document reviewal is inherently time-consuming. The more content is created, the more time is needed to manually review and proofread it.
- Manually proofreading content is error-prone. As document sizes increase, so do opportunities to introduce errors.
- With every inspection stage, new changes are being introduced. This increases the chance of error as not only does the original content need to be checked, new additions need to be as well.
- Manually proofreading is decentralized with many people looking over a single document with different methods and processes. This can lead to fragmented, confusing, and unfinished inspections.
Any error that slips through in any stage of content production costs companies money as these errors can result in expensive recalls. Errors also cause duplicated work, revisions, and significant delays in go-to-market time with every interruption in the process causing potential additional financial losses.
Most proofreading challenges will occur in the development and approval stages of content. These stages are non-linear and fragmented as there is a lot of back and forth to ensure that all errors are being found and corrected accordingly. However, with every hand-off and every new reviewal process, there is the possibility that new errors are introduced, further complicating the process.
When products and content have finally been approved, the proofreading and document reviewal process is not over. When products make it to market, during the monitoring phase, if new discoveries are made, changes in artwork and content are needed. Updates need to be made to the prescribing method, results claims, side effects, and regulatory requirements to name a few.
With all-new changes and adjustments, the product proofreading process needs to be repeated and the complex lifecycle once again begins. With such intricate and challenging steps that need to be undergone, companies cannot risk errors slipping through due to the multitude of disadvantages that come with manual proofreading.
Thus, regulated industries are turning towards automation as they focus strongly on operational efficiency.
The creation of digital and printed content includes the following steps
- Plan - what products need to be developed, produced, and distributed? What accompanying tests, trials, and documentation need to be undergone and created?
- Initiate - begin the process of creating products and start the necessary steps to get products to the production phase.
- Develop - create, produce and develop products and accompanying content.
- Approve - go through tests and trials to approve products and content for markets.
- Release - products go to market and to consumers.
- Monitor - monitor how the product functions and if there are any negative side effects.
- Change - make necessary changes to ensure the product is accurate and functioning properly.
Automation as a Solution
Bringing a pharmaceutical product to market is one of the most necessary, complex, and highly sophisticated processes in business globally, however, there is one major piece that is broken.
Many large global corporations have yet to implement efficient and effective workflows that allow them to proofread content in record time with complete accuracy. They still heavily rely on manual and labor-intensive proofreading processes that are prone to costly mistakes. Taking hours, days, or even weeks to check a single document is not a probable solution for large corporations that produce and distribute millions, if not billions, of products a day.
There is huge potential for these processes to be optimized and streamlined for maximum efficiency.
The documentation review process is broken.
The solution, thankfully, is simple, and it lies within using automated quality control to look over all content and artwork with complete ease, at lightning speed, and with increased productivity and accuracy.
To keep up with increasing global consumer demands, pharmaceutical companies are creating almost immeasurable amounts of products, each with its own packaging, labeling, and written instructions. All of this content needs to be meticulously overlooked, carefully checked, and proofread to ensure that the final product is completely error-free. Manually proofreading these neverending amounts of content is simply not an efficient solution for organizations that need to keep up with growing demands.
Automated proofreading software offers a foolproof solution to ensure that all this generated content is checked and proofread to perfection.
This advancement in proofreading processes eliminates the need for manual document inspections and leaves it up to technology. The software conducts digital checks looking for discrepancies in text, spelling, graphics, color, and more.
While this could be done manually, the software finishes proofreading processes in a fraction of the time and ensures that your work is 100% accurate before going to print, avoiding the need for multiple inspections downstream.
This advancement in technology ultimately increases productivity and workflow efficiency and offers endless benefits unmatched by manual inspections. Not only does automated proofreading software help ease the proofreading process, it simply makes proofreading better, optimizing workflow processes and allowing for the completion of proofreading tasks with complete and utter ease.
The GlobalVision Advantage
GlobalVision’s innovative automated solutions have three decades of solving problems for Enterprise Pharma behind them.
After over thirty years of working with some of the largest and most successful pharmaceutical brands globally, GlobalVision has listened to customers, learned, tailored, and innovated, to create unprecedented market-leading automated proofreading and inspection technology that has transformed the life sciences industry.
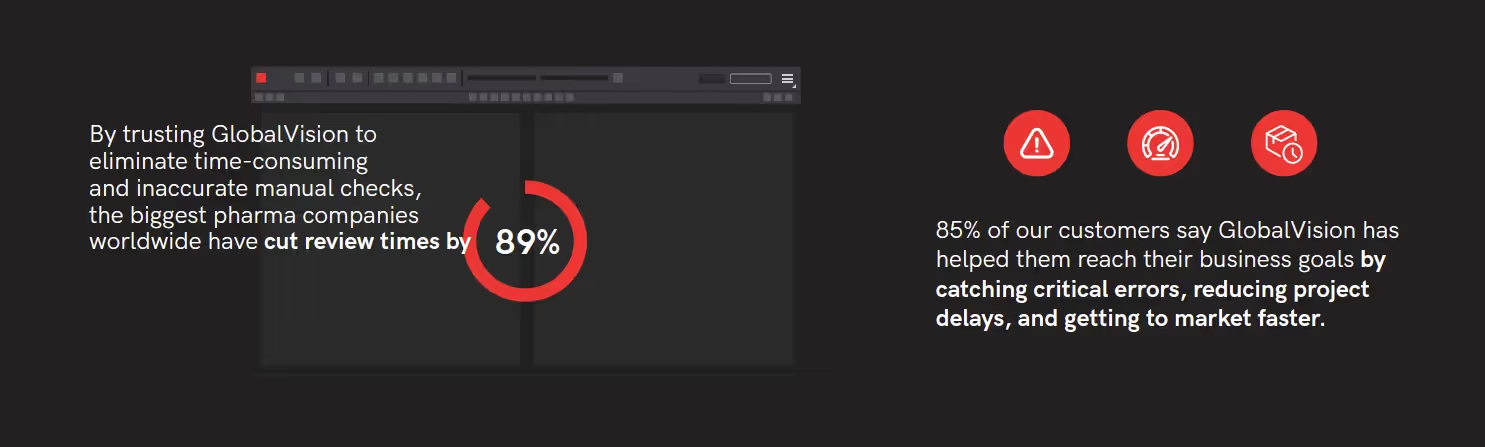
By trusting GlobalVision to eliminate time-consuming and inaccurate manual checks, the biggest pharma companies worldwide have cut review times by 89% - getting critical health care products into the hands of consumers faster, and in doing so, are confident their documentation is 100% accurate and compliant.
Using GlobalVision’s proofreading solutions, companies can expect to compare documents at rates of 1000 characters per second. In contrast, the average reading speed is 200 to 250 words a minute, and the average person in business reads no faster than people did 100 years ago.
What can you expect from GlobalVision?
Increased efficiency. With automated quality control, companies can expect faster reviewing and inspection processes while increasing the accuracy of their artwork and content. GlobalVision’s automated solutions have already helped many internationally renowned pharmaceutical companies streamline their workflows to help them create accurate content and artwork, every time.
Bristol-Myers Squibb (BMS) Delivers Consistent Quality with GlobalVision
At BMS Shanghai, GlobalVision is used as part of a two-fold system. The process starts by storing the BMS-approved sample in a computer ahead of receiving the printed proofs. Once the print proofs are received, they are evaluated against the approved sample to detect defects in text, graphics, and other elements. Additionally, the Quality Control Department uses GlobalVision to inspect incoming packaging materials such as cartons. Batch by batch, the packaging is compared to the approved samples to catch any differences between the two. As a result of the automation, the Quality Control Department has found that its revisions could be completed faster and more effectively.
With batches of 500 cartons and 1250 labels, BMS appreciates the fact that they can now save an extensive amount of time when running inspections.
We can save over 15 minutes per batch when testing incoming samples with GlobalVision.
Xu Danqing, Quality Control Specialist, Bristol-Myers Squibb Shanghai
Conclusion
While traditionally manual inspections were the only method used to proofread content, modern-day technology has allowed for faster and more efficient solutions to get the job done right. With endless benefits and countless advantages to your team’s workflow, automated quality control is the leading solution for enterprise pharma companies that deal with large amounts of sensitive and highly regulated documentation and content.


